Cancer Therapy Nanobots Successfully Seek & Shrink Tumors
Scientists at the Arizona State University in collaboration with those at the National Center for Nanoscience and Technology (NCNST) have now successfully designed and developed an autonomous DNA robot programmed to transport payloads and present them specifically in tumors using DNA Origami.
“We have developed the first fully autonomous, DNA robotic system for a very precise drug design and targeted cancer therapy,” said Hao Yan, director of the ASU Biodesign Institute’s Center for Molecular Design and Biomimetics and the Milton Glick Professor in the School of Molecular Sciences.
“Moreover, this technology is a strategy that can be used for many types of cancer, since all solid tumor-feeding blood vessels are essentially the same,” Yan said.
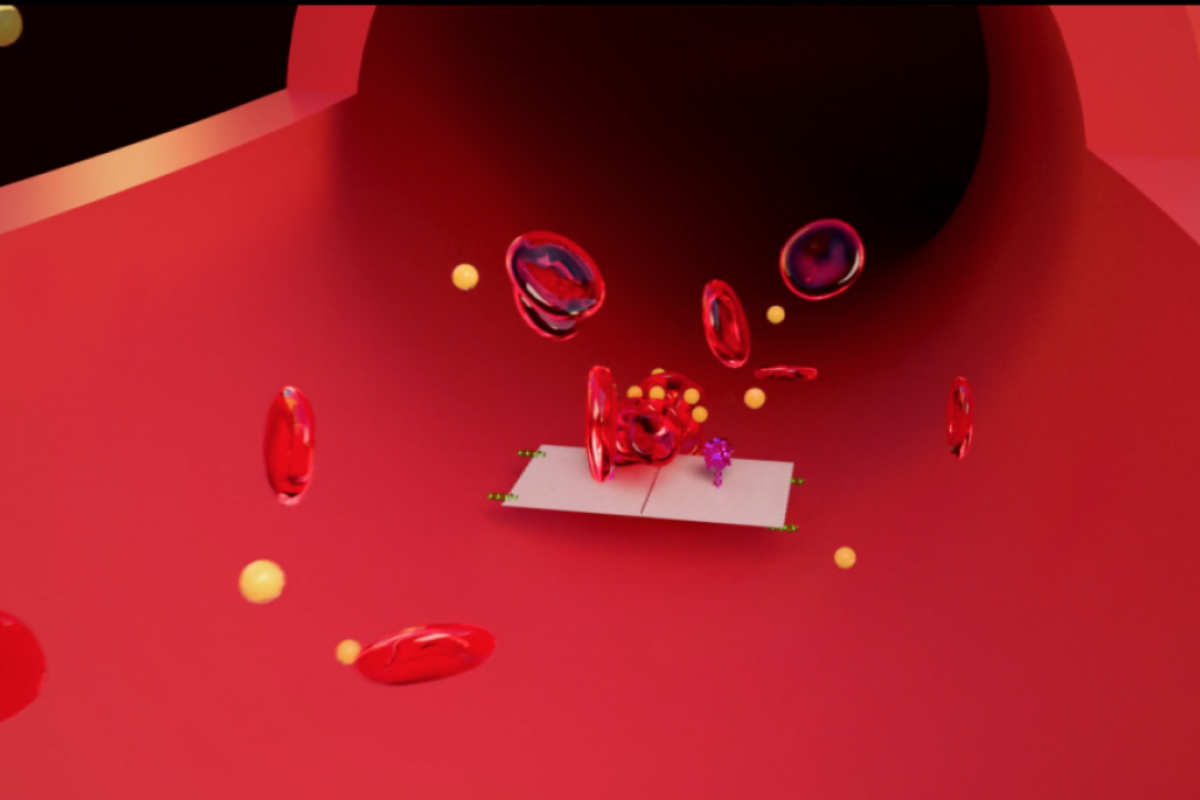
In the demonstration of their work, the mini robots were seen to be able to cut-off the blood supply to breast cancer, melanoma, ovarian and lung cancer tumors in mice. After just two weeks of treatment, the researchers reported that the tumor tissue was shrinking.
Blood-clotting drugs, normally used to
tackle minor bleeding rather than as cancer treatment, were carried by nanobots, made from origami-folded DNA sheets, to shut off the blood supply to tumours throughout the body. Starved of their blood supply, tumours began to shrink and the cancer’s ability to spread and grow in new sites also appeared to be reduced, doubling the life expectancy or removing tumours entirely in some mouse cancers.This demonstration, which marks a major step towards the implementation of nanobot drug delivery in medicine, could pave the way for delivering toxic chemotherapy drugs with reduced side effects, among a host of other uses.
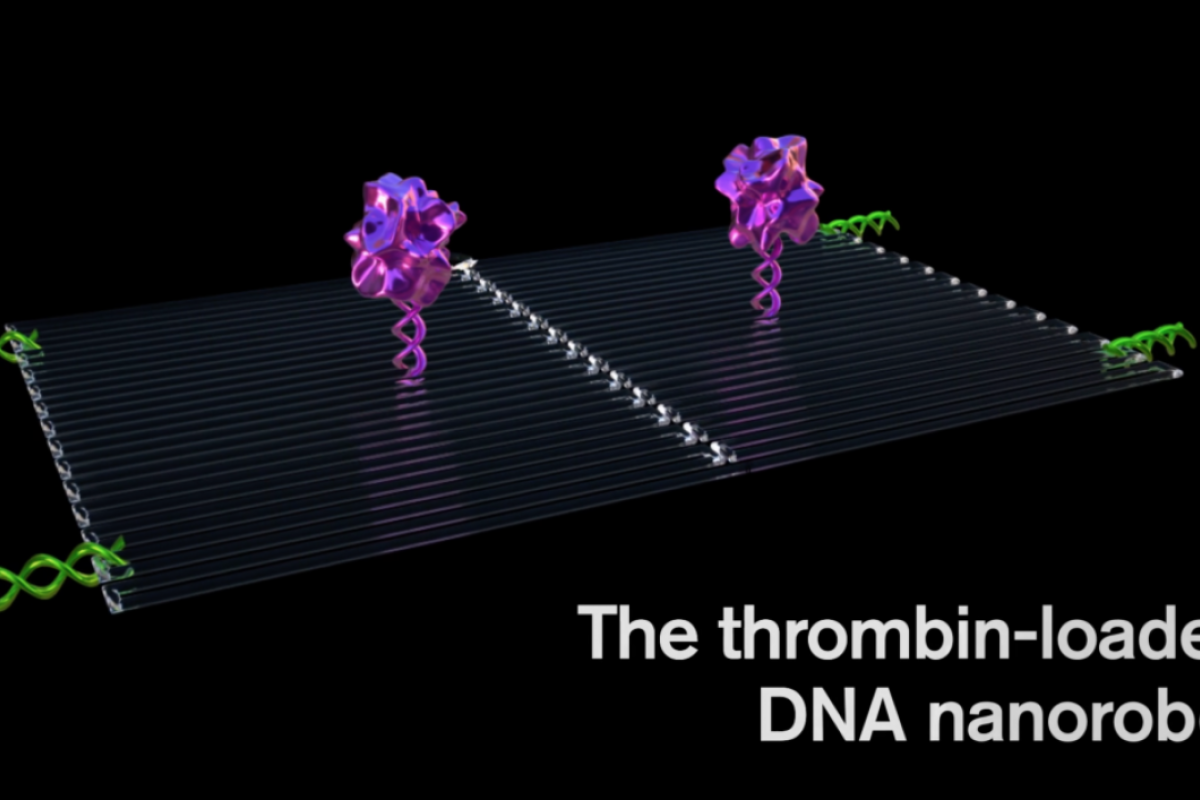
Using “DNA origami”, the researchers folded a DNA nanorobot into a tube structure containing thrombin on the inside. The tubular nanorobots were additionally programmed to open up and release their payload when locked onto nucleolin, a protein specific to the surface of blood vessels feeding tumor cells.
Each nanorobot is created from a flat, rectangular DNA origami sheet, just 90 nm by 60 nm in area, and just 2 nm thick. Four thrombin molecules are attached to the origami sheet surface, which is then rolled up into a hollow tube with the thrombin molecules protected on the inside. The tube structure is held together by fastener strands that include DNA aptamer molecules designed to nucleolin, a protein specifically expressed on tumor-associated endothelial cells. The final construction is a hollow, tube-shaped DNA nanorobot with a diameter of about 19 nm and a length of about 90 nm.
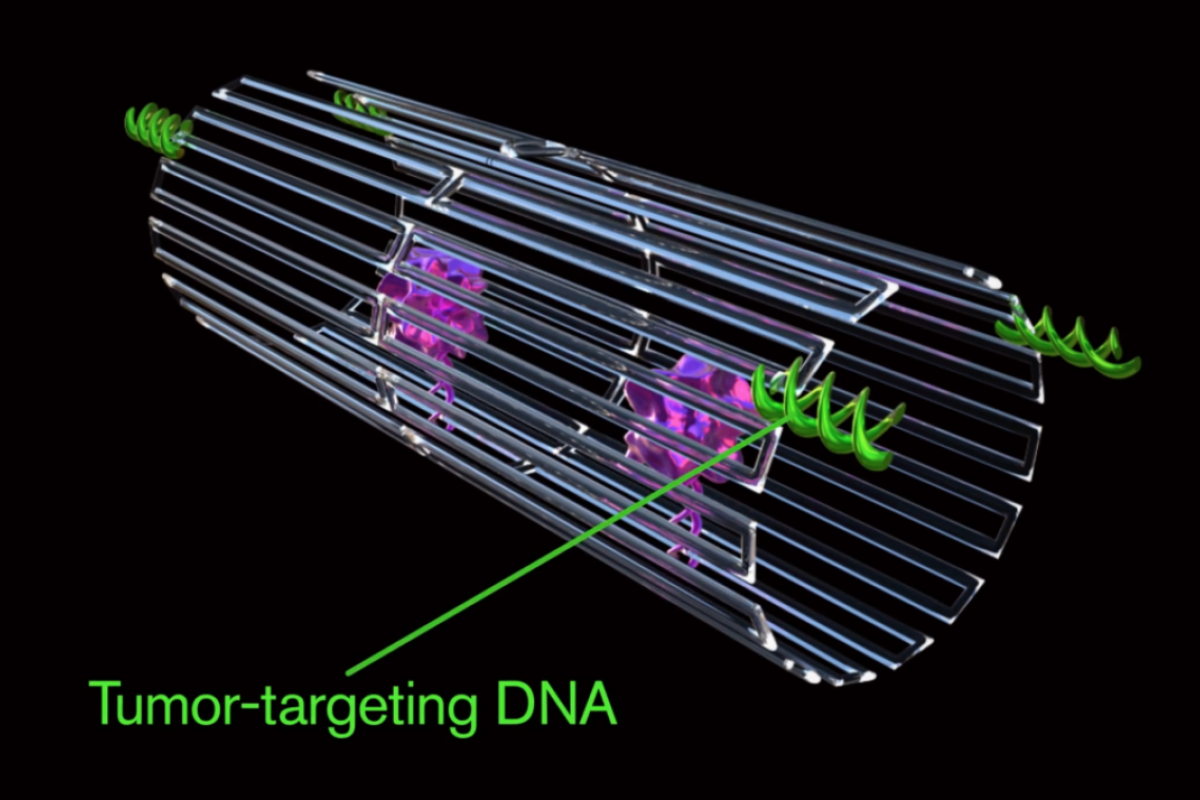
When the team injected the nanorobots into mice carrying human breast cancer tumor cells, the bots managed to successfully latch onto the blood vessels around the tumor and cause extensive blood clots within the first 48 hours. This caused the tumor to shrink and start to die.
“The thrombin delivery DNA nanorobot constitutes a major advance in the application of DNA nanotechnology for cancer therapy,” claims Yan. “In a melanoma mouse model, the nanorobot not only affected the primary tumor but also prevented the formation of metastasis, showing promising therapeutic potential.”
“DNA nanorobotic systems, such as the one we describe here with targeting and triggered release properties, may inspire the design of novel cancer therapeutics modified with different targeting ligands to mediate delivery of multiple biologically active payloads, such as short interfering RNA (siRNA), chemotherapeutics or peptide drugs,” the authors suggest. They are now looking for partners to develop the technology for clinical applications.
“I think we are much closer to real, practical medical applications of the technology,” Hao Yan states. “Combinations of different rationally designed nanorobots carrying various agents may help to accomplish the ultimate goal of cancer research: the eradication of solid tumors and vascularized metastases. Furthermore, the current strategy may be developed as a drug delivery platform for the treatment of other diseases by modification of the geometry of the nanostructures, the targeting groups, and the loaded cargoes.“






























Nanorobot is good for melanoma and other tumors in mouse. I have cancer as probably millions of people have and I am glad that after 70 years of cancer research we can finaly say that in the last few years we have cancer cure for mouse. They are more lucky than people. Most people like cats and dogs more than mouse. It will be better to have cancer drugs for dogs and cats as they are
our pets. But scientists have almost whole 21st century in front of them for research….No rush.