Inhalable anti-COVID-19 antibody decreases viral load in hamsters
TFF Pharmaceuticals got $14 million in a Series A round in 2018 with the goal of developing innovative inhalable therapies for asthma, chronic obstructive pulmonary disease, and other severe lung diseases using its dry-powder drug-delivery technology. Then the COVID-19 pandemic struck, and the firm saw an opportunity to use their technology against the SARS-CoV-2.
As a result, the Austin, Texas-based firm collaborated with California-based Augmenta Bioworks to create an inhalable monoclonal antibody to treat COVID-19 infection. The drug decreased viral load in hamster models one day after infection with COVID-19. Both the companies reported their findings on the journal preprint site bioRxiv. They intend to begin human trials in 2022.
The inhalable drugs being developed by TFF are based on a “thin, freezing film” technology authorized from the University of Texas, Austin. It concentrates on biologics and small molecules that have yet to be developed as inhalable drugs. The firm currently has two drugs in phase 1 trials – TFF for invasive pulmonary aspergillosis and TFF TAC-LAC, a dry-powder version of tacrolimus, a generally utilized immunosuppressive drug in organ transplants.
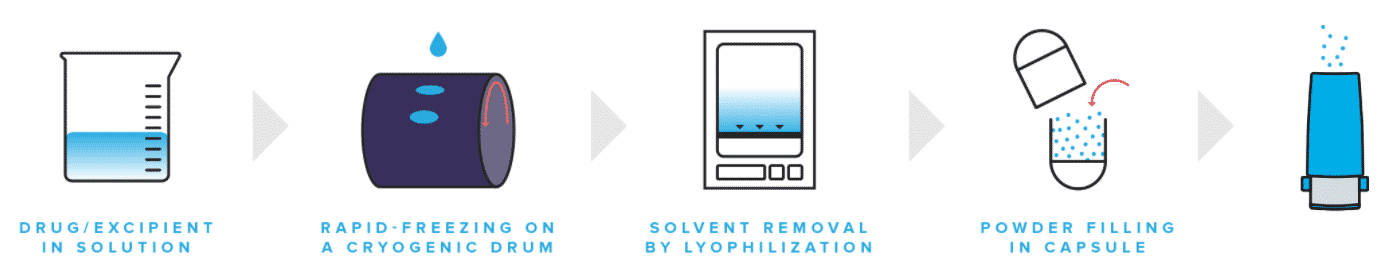
The COVID-19 drug, AUG-3387, is an entirely human
antibody isolated and examined by TFF and Augmenta for its capability to bind to the SARS-CoV-2 spike protein. According to the firms, the antibody neutralized SARS-CoV-2 and its delta variant in cell studies.The scientists administered a dry-powder version of the drug to hamsters one day after being exposed to SARS-CoV-2. They discovered that the viral load in the animals decreased based on the dosage.
There are antibody treatments for COVID-19 in the market presently, like Gilead’s remdesivir, which is used in roughly half of the hospitalized patients. However, this and other approved treatments must be introduced in healthcare centers, which TFF and Augmenta hope to eliminate.
The researchers mentioned that while antibody therapeutics presents a clear therapeutic advantage for decreasing hospitalization, the mode of administration remains to place a hardship on the healthcare system. So, drug administration to patients in an outpatient environment without the necessity for specific infusion centers would be a significant step forward.
TFF and Augmenta’s study came just days after Merck and Ridgeback Biotherapeutics declared that their oral COVID drug, molnupiravir, reduced the possibility of hospitalization or mortality by half. An FDA advisory committee will meet on November 30 to consider a EUA for the drug; however, some Wall Street analysts predict it will be a blockbuster.
Last week, Ronny Gal, a Bernstein analyst, estimated that the entire global market for oral antivirals would be valued at $6 billion, even after COVID-19 is downgraded from a pandemic to an endemic virus.
TFF and Augmenta have stated that they intend to develop AUG-3387 for two purposes: treating coronavirus-infected people who have not yet been hospitalized but stand a high threat of critical complexities and preventing the disease in high-risk people.
Also Read:
- Ph.D. Mandatory From 2023 For Assistant Professors: UGC
- Comprehensive Atlas Of Brain Cells – A Neuroscience Dream
- Can We Predict Next Virus Spread From Animals to Humans With AI?
- Uncertain Monsoon In India
Download Biotecnika App for more Updates on Life Sciences, Job vacancies, CSIR-NET, GATE, and a lot more.
Keyword: Inhalable Anti-COVID-19 Antibody Decreases Viral Load In Hamsters