Human Cotransporter Protein Structure Solved by Researchers
In research that could someday improve treatments for epilepsy, UT Southwestern researchers have published the first 3D structure of a member of a large family of human cotransporter proteins that carry charged particles, ions, across the cell membrane.
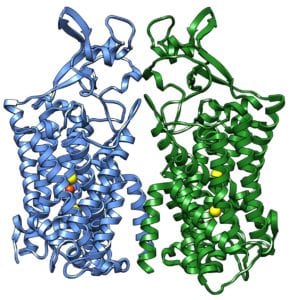
The potassium chloride co-transporter 1 (KCC1) structure solved in this research carries positively charged potassium ions (K+) and also negatively charged chloride (Cl–) ions across the cell membranes to help regulate the volume of the cell. The protein structure solved is one of a large family of co-transporters found in many of the body’s tissues, particularly in the kidneys and the brain.
Despite extensive researches of cotransporters, the lack of high-resolution structures has hindered a deeper understanding of their actions. The researchers solved the structure using cryo-electron microscopy. Cryo-EM is an advanced technology in which samples are frozen at very low temperatures at speeds that prevents the formation of the ice crystals.
Mutations in this family of protein cotransporters can lead to diseases such as hereditary epilepsy, including one form that starts in infancy, said Dr. Xiao-chen Bai, the corresponding author of the Science study. Drugs that target co-transporters are currently used as diuretics to treat high blood pressure.
The cryo-EM method was the only way to determine the structure of an integral membrane protein such as this one. The team hopes this structure will facilitate the design of drugs that target this protein, said Dr. Bai, Assistant Professor of Biophysics and Cell Biology and a Virginia Murchison Linthicum Scholar in Medical Research as well as a Cancer Prevention & Research Institute of Texas (CPRIT) Scholar.
The proteins on the cellular membrane have been particularly resistant to X-ray crystallography. X-ray Crystallography is formerly considered the gold standard in structural biology technology before the cryo-EM method.
In the cryo-EM method, samples are viewed using robot-assisted microscopes that are twice as tall as a person. These microscopes contain high-tech electron detectors that work with powerful computers to record multiple images & apply advanced algorithms to interpret the data. UT Southwestern’s CryoEM Facility operates 24 hours a day, 7 days a week.

Other study participants included scientists at Vanderbilt University in Nashville & from China’s Zhejiang University School of Medicine, Tianjin University, and Wuxi Biortus Biosciences Co. Ltd. One of the corresponding authors of the study is Dr. Jingtao Guo of Zhejiang University School of Medicine, who began the project while a postdoctoral researcher in the UTSW laboratory of Dr. Youxing Jiang, Professor of Physiology & Biophysics. Dr. Jiang holds the Rosewood Corporation Chair in Biomedical Science & is a W.W. Caruth, Jr. Scholar in Biomedical Research at UT Southwestern and an Investigator of the Howard Hughes Medical Institute.
The research study received funding from China’s Ministry of Science and Technology, the National Natural Science Foundation of China, Zhejiang Provincial Natural Science Foundation, the Fundamental Research Funds for the Central Universities, and Zhejiang University, and from CPRIT, The Welch Foundation, the National Institutes of Health, and the Leducq Foundation.
The authors report no competing financial interests.






























