Clinical Data Management Career Guide: Salary Trends, Job Opportunities & Career Growth
Do you know the fact that Clinical research is the backbone of modern medicine?
Yes, from life-saving drugs to therapies, every innovation happening in medicine depends on these high-quality and reliable data. Today, clinical data management (CDM) is the center of the whole clinical research process. CDM is where the clinical data is collected, cleaned, and analyzed. With this, we make sure that the data is accurate, integrated, and matches all regulatory requirements.
Clinical Data Management is one of the most demanding job fields in 2025. With recent developments in AI, machine learning, and decentralized clinical trials, the demand for CDM has increased drastically. It’s no longer about spreadsheets or manual checks. This field, with a blend of science and data, is also offering new career opportunities for life science professionals.
If you want to be part of this innovative field, then here we are to help you understand Clinical Data Management. Let’s talk about the salary trends, career opportunities, and growth in this field.
- What Is Clinical Data Management?
Let’s first start by understanding what CDM is. It is the process of collecting, validating, and organizing data produced during clinical trials. As a CDM professional, you are responsible for ensuring that every piece of trial data is accurate, consistent, and regulatory-compliant. You will be transforming raw numbers into reliable insights that will be used for drug approvals and protecting patient safety.
You will be the guardian of data integrity, where you will be an investigator, storyteller, and compliance expert.
- Salary Trend – Clinical Data Management Career Guide
Now that you have understood the actual role of what a CDM does, and have made up your mind to build a promising career in this field, let me provide you with some current industry hiring trends:
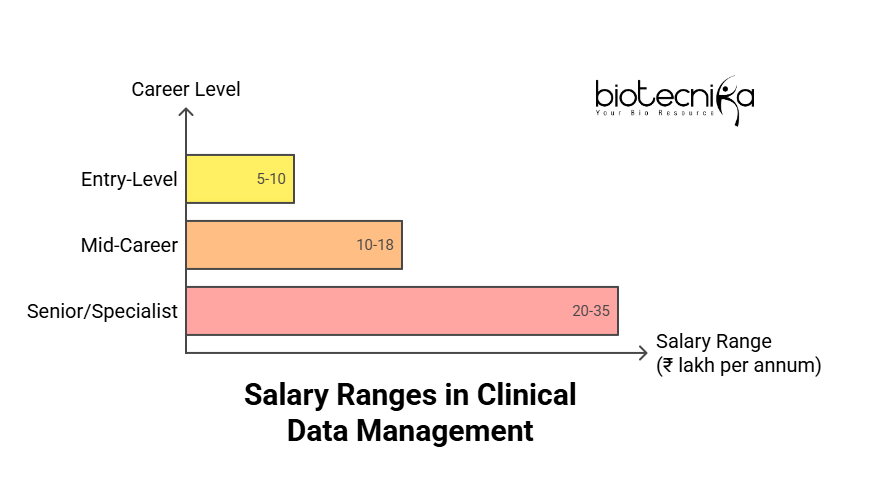
For entry-level roles, the typical salary package starts from ₹5–10 lakh per annum. Academic background, technical skills, and certifications together can act as major factors in influencing your payroll.
Whereas, a mid-career professional will earn somewhere between ₹10–18 lakh per annum. At this stage of your career, skills such as SAS programming, regulatory documentation, and managing multi-country trials play an important role in your career growth.
As you grow in your career, you can earn ₹20–35 lakh per annum as a senior and niche specialist. At the same time, you can be in leadership positions at global pharma firms, large CROs, or tech-enabled healthcare companies.
So, if you are planning to start your career in Clinical Data Management in 2025, then let me tell you this is a golden opportunity. The industry is blooming, and with the right skills, you can climb the success ladder faster than ever before.
- Job Opportunities
If you have the skills, the opportunities are knocking louder than ever. The CDM job market in India is expanding rapidly than traditional pharma companies. This is due to Contract Research Organizations (CROs), healthcare IT firms, and hospitals hunting talented candidates. Do you know what the best part is? There is a wide range of jobs that allow you to find the one that aligns perfectly with your strengths and career aspirations.
List of Top CDM Job Roles in India
- Clinical Data Coordinator (entry-level)
- Data Analyst / Programmer
- CDM Project Manager
- Standards Consultant
- Systems Lead
The field offers both remote and hybrid job opportunities. This was possible due to tools such as cloud-based collaboration platforms, analytics dashboards, and electronic data capture (EDC) systems.
Along with this, the industry is expanding too.
Let me give you an example of how this industry is growing before we discuss the career path. The biotech giant, AstraZeneca, has recently announced an ₹166 crore expansion of its Bangalore Global Hub. This expansion will create around 400 jobs in AI-driven R&D, digital health, and most importantly, clinical research analytics. This shows the large-scale investment in India for bringing more CDM jobs.
- Career Growth
Remember, your first CDM job is just he starting line. From entry-level coordinator to strategic decision maker, this field offers a well-defined and flexible career path. As technology is advancing, your ability to adapt and lead will determine your success rate in the industry. This will also indicate how much influence you will have on the future of clinical research.
What CDM career path might look like?
As an entry-level clinical data coordinator, you will learn data entry, cleaning, and query management. Then, as a Data Analyst or Programmer, you will gain expertise in SAS, SQL, and statistical reporting.
Further, as Data Manager or Project Manager, you will have to oversee trial databases and cross-functional coordination.
When you reach a Standards Consultant or Systems Lead position, you will bridge data standards (CDISC, SDTM) with enterprise clinical systems. The Certified Clinical Data Manager (CCDM) certification from the Society for Clinical Data Management (SCDM) stands as the industry standard.
With CDM now merging into Clinical Data Science, professionals are not just “babysitting data.” They are interpreting data, predicting trial risks, and contributing to strategic decisions across safety, operations, and analytics.
- Where the Jobs Are: Clinical Data Management Career Guide
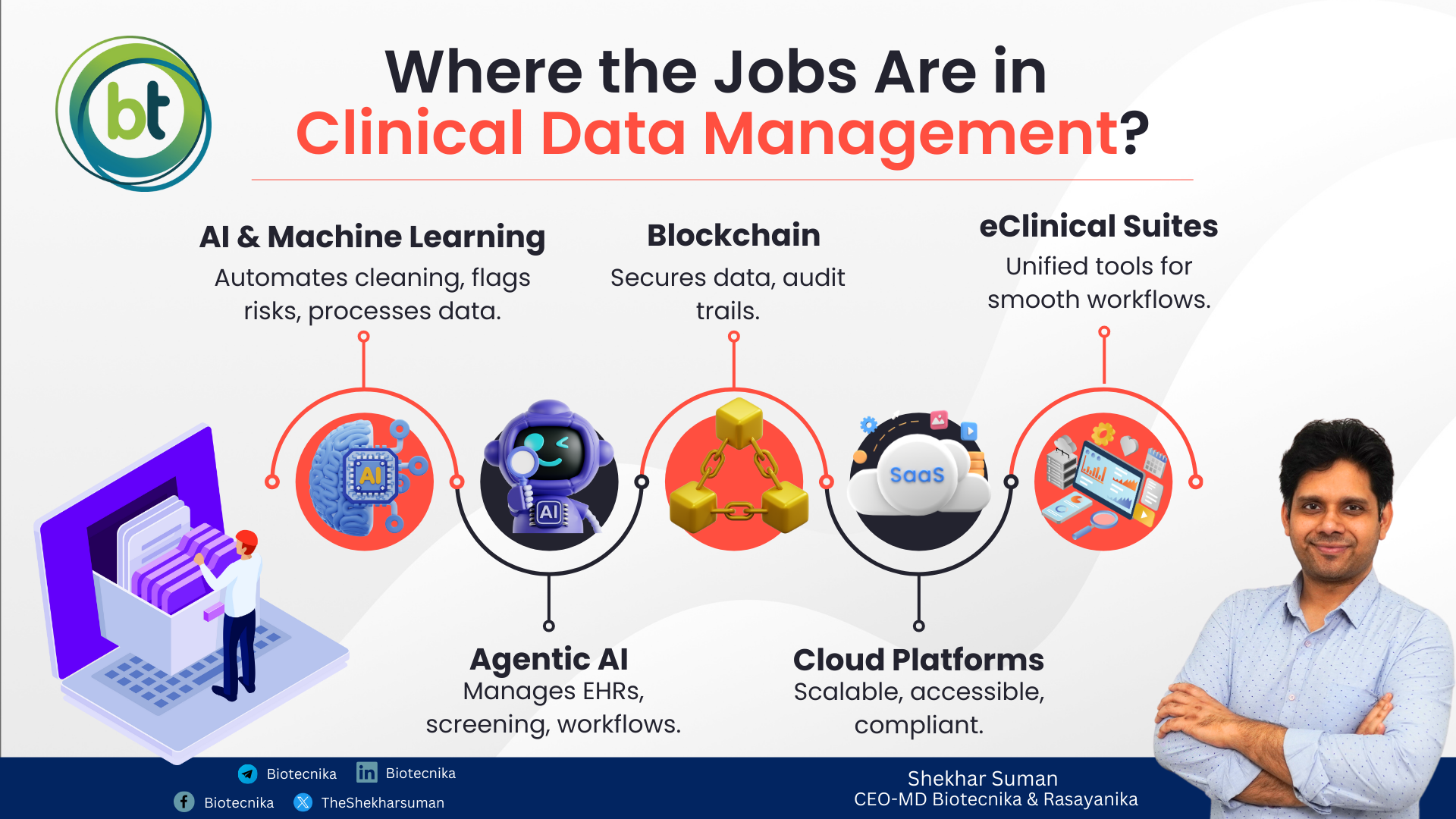
Today, clinical trials are not just run by people, but also powered by technology. AI, blockchain, and integrated eClinical platforms are transforming how data is collected, validated, and analyzed. Staying ahead of these trends helps you be a leader in the industry rather than being a regular participant.
Let us know about where the jobs are now:
- AI & ML: Responsible for automating data cleaning, flagging anomalies, predicting risks, and processing unstructured data with NLP.
- Agentic AI: Usage of AI agents for autonomously reviewing electronic health records (EHRs), screening participants, and managing workflows from site to sponsor.
- Blockchain: Responsible for securing trial data, ensuring tamper-proof audit trails.
- Cloud-Based Platforms: Responsible for offering scalability, remote accessibility, and compliance in one environment.
- All-in-One eClinical Suites: Responsible for integrating eConsent, EDC, CTMS, eCOA, and IRT into unified platforms for smoother workflows.
- Challenges
Challenges are a part of every success story, and Clinical Data Management has also c has its own challenges:
- Trial designs such as those for oncology and rare diseases are increasingly complex.
- Managing vast real-world evidence (RWE) datasets can be difficult.
- Navigating global regulatory challenges
- Shortage of professionals trained in AI + CDM.
The solution for all these challenges is hidden in continuous learning, hands-on training, and having project-based experience that are essential for staying ahead of the competition.
- Right Training
In CDM, your current skill set is just your starting toolkit. To succeed in the 2025 job market, you need to keep upskilling. New technical competencies, refining your soft skills, and staying informed about regulatory and technological shifts will help anyone stand out from the crowd. The more you learn, the more valuable you will become to employers and the scientific community.
To stay ahead in CDM, focus on –
Technical Skills to Master:
- Programming sector: Python, SAS, SQL
- Regulatory Standards: CDISC, SDTM, ADaM
- AI&ML Basics & NLP Workflows
Soft Skills:
- Regulatory knowledge
- Clear, concise communication
- Adaptability in trial environments
Preferred Certifications & Internships:
- Gain hands-on experience through internships or live projects
- Earn recognized certifications in CDM, SAS, or clinical research
- Build a portfolio showcasing technical and analytical capabilities
Clinical Data Management Career Guide
- How Biotecnika Can Help You
Clinical data management can feel overwhelming, especially when it comes to the rising demand for both technical expertise and regulatory knowledge. This is where our Clinical Data Management Training Program: Basic to Advanced With Hands-on Experience becomes a game changer.
Why Choose Biotecnika?
- Covers all CDM processes
- Hands-on training with real-world case studies and datasets.
- Work on a Real Project to give you practical industry exposure.
- Get Trained by industry experts
- Learn emerging technologies and regulatory tech.
Who should enroll?
- Life science students (BSc, MSc, BPharma, MPharma, Biotechnology, Microbiology, Genetics, Bioinformatics).
- Fresh graduates who are looking for their first industry role.
- Working professionals who are aiming to switch to CDM or advance their careers.
With the right training, you can bridge the gap between classroom learning and industry needs. It prepares you for the 2025 booming CDM market.
Clinical Data Management in 2025 is no longer just about entering trial data. It’s about shaping the future of medicine with technology and precision.
So, if you’re passionate about life sciences but also love data and technology, this is your golden ticket. Start learning today. And within a few years, you could be leading projects that bring breakthrough medicines to patients worldwide.
Because every new therapy needs data, and every data needs a manager. That manager could be you. Get ready to embrace this innovative and impactful journey of Clinical Data Management in 2025.