Coronavirus vaccines manufactured in India
The whole globe remains in the middle of a COVID-19 pandemic. Vaccines save millions of lives each year; we can eliminate COVID-19 only by using vaccines. Vaccines work by training and developing the body’s immune system to detect and fight off pathogens. Presently, there are over 50 COVID-19 vaccine candidates in trials across the world. The established vaccines will be provided to the frontline workers.
Now let’s discuss the Coronavirus vaccines and their types manufactured in India
- Covishield
| Developed By | University of Oxford and AstraZeneca in collaboration with the SII |
| Vaccine Type | Modified chimpanzee adenovirus vector |
| Efficacy | DCGI: 70.42% overall |
| Storage Temperature | 2-8°C |
| Dosage | Two doses (Gap 2.5-3 Months) |
| Routes of administration | Intramuscular injection |
Covishield is also known as s ChAdOx1 nCoV-19, or AZD1222, developed by Oxford University in partnership with AstraZeneca. Its manufacturing and trial partner is the Serum Institute of India, Pune, and ICMR.
This vaccine uses a replication-deficient chimpanzee viral vector based on a weakened version of adenovirus that causes infections in chimpanzees and consists of the genetic material of the coronavirus spike protein, which helps the virus to bind with the human cells. The modified chimpanzee adenovirus can’t replicate, hence do not cause infection, and rather serves as a vector to transfer the coronavirus spike protein.
Like most of the vaccines, the Covishield produces the mimic spike protein that causes an immunological response, which would finally prime the immune system.
Covishield is approved in India, Argentina,
El Salvador, Mexico, Bangladesh, and the Dominican Republic regulatory authorities for emergency use.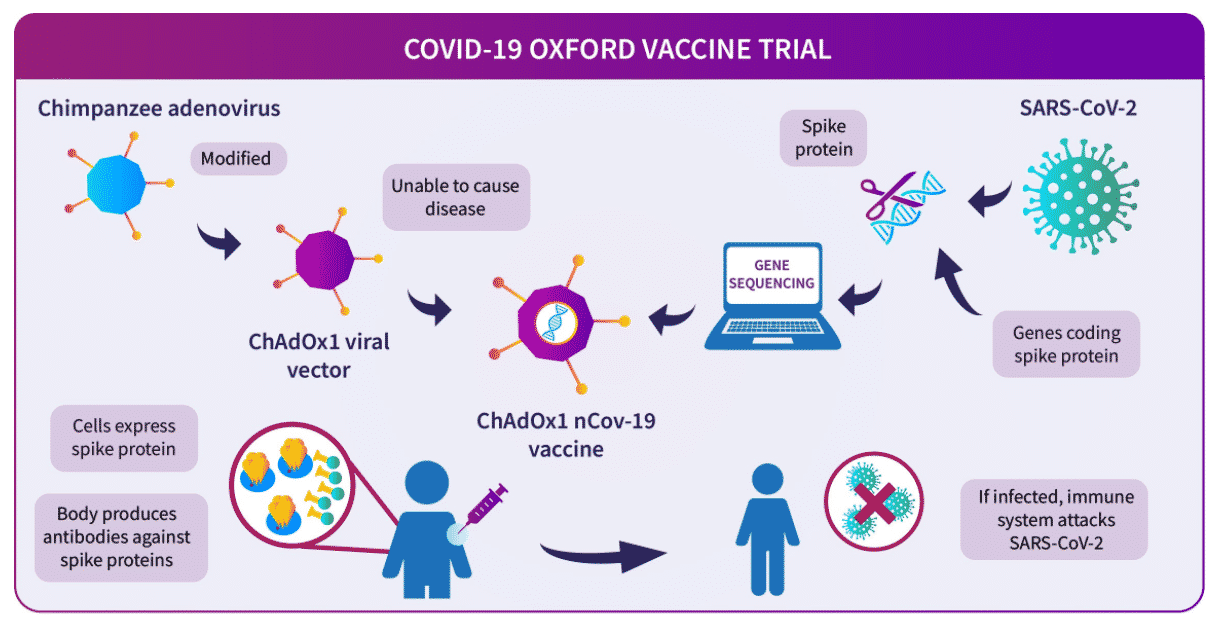
The vaccine will be available for frontline workers and older people by February 2021; however, the general public of the age group will have to wait until April 2021. Likely, the Covishield vaccine would be available at ₹200 per dosage.
The pact was made between the Indian government and SII for a purchase order of 11 million doses.
2. Covaxin
| Developed By | Bharat Biotech in association with ICMR and NIV |
| Vaccine Type | Inactivated whole virus |
| Efficacy | N/A |
| Storage Temperature | 2-8°C |
| Dosage | Two Doses (0, 14 Days) |
| Routes of administration | Intramuscular injection |
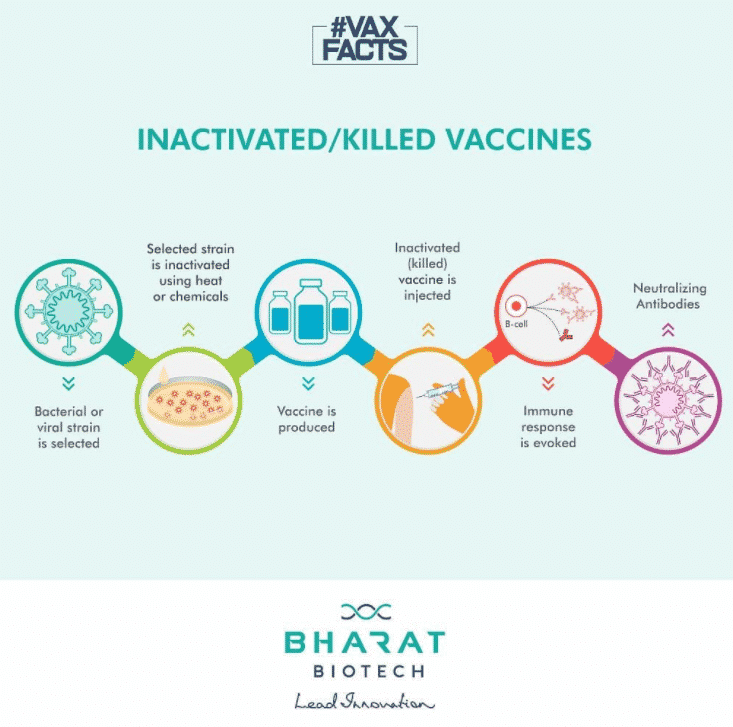
Covaxin is India’s first indigenous COVID-19 vaccine. It is an inactivated vaccine that suppresses the virus’ capability to duplicate yet keeps it unimpaired so that the immune system can still recognize it and create an immune reaction. Covaxin helps in increasing the production of antibodies in the host body.
This vaccine can target the new variant of SARS-CoV-2 that was recently detected in the UK. This vaccine produces a strong immune response and in-vitro viral neutralization. Both homologous (vaccine virus strain) and heterologous (divergent) SARS-CoV-2 strains were neutralized using this vaccine, as per the vaccine-induced antibody responses recorded.
After successfully finishing the interim report from the Phase 1 & 2 clinical trials of COVAXINTM, it was approved by the Drugs Controller General of India for Phase 3 clinical trials in 26,000 participants in over 25 centers across India.
The cost of Covaxin will most probably be ₹295. The Indian Government has asked the manufacturing company to provide around 55 lakh dosages at 12 centers before 14 January 2021.
3. ZyCoV-D
| Developed By | Zydus Cadila |
| Vaccine Type | Plasmid DNA vaccine |
| Efficacy | N/A |
| Storage Temperature | 2-8°C |
| Dosage | N/A |
| Routes of administration | Intradermal route |
Zydus Cadila, Ahmedabad ZyCoV-D established the DNA vaccine system in India, known to show enhanced vaccine stability, demanding lower cold chain requirements, making the vaccine ideal for access to remote areas of the nation.
Zydus Cadila received approvals from the DCGI to initiate Phase III Clinical Trial of ZyCoV-D.
The manufacturing process is easier while using this platform, and the platform can be altered in just a few weeks if the virus gets mutated.
The plasmid DNA, when introduced into the host cells, would be translated into the viral protein and will evoke a robust immune reaction, which helps in protecting from COVID-19 and viral clearance.
As per phase I and II clinical trials, ZyCoV-D is safe, immunogenic, and well-tolerated.
4. Biological E
| Developed By | Biological E |
| Vaccine Type | Subunit vaccine |
| Efficacy | N/A |
| Storage Temperature | N/A |
| Dosage | N/A |
| Routes of administration | Intramuscular injection |
Biological E has started Phase I/II clinical trials to assess the safety and immunogenicity of its COVID-19 subunit vaccine candidate after it was approved by DGCI.
The clinical trials will also analyze the capability to generate an immune response of the vaccine candidate, which has the Receptor Binding Domain of the Spike Protein of SARS-CoV-2 at three dose levels adjuvanted with CpG 1018 plus alum.
The human trials will include 360 healthy individuals of 18-65 years. The vaccine will be given as two dosages 28 days apart.
The ICMR, in partnership with Biological E Ltd, developed “highly purified antisera” developed by injecting inactivated SARS-CoV2 in horses, which can be used for treating prophylaxis and COVID-19.
5. Mynvax
| Developed By | Mynvax -IISc |
| Vaccine Type | Protien based vaccine |
| Efficacy | N/A |
| Storage Temperature | 37º C |
| Dosage | N/A |
| Routes of administration | N/A |
Mynvax’s COVID-19 vaccine uses a unique method involving a protein-based vaccine. This vaccine can be stored at room temperature for 1 month without losing its effectiveness. This eliminates the need for cold chains. The vaccine is now in the phase for formal safety and toxicity studies, process development, human clinical trials, and manufacturing.
This heat-tolerant vaccine can endure 70º C for nearly 16 hours, and the freeze-dried form of the vaccine can withstand 100º C.
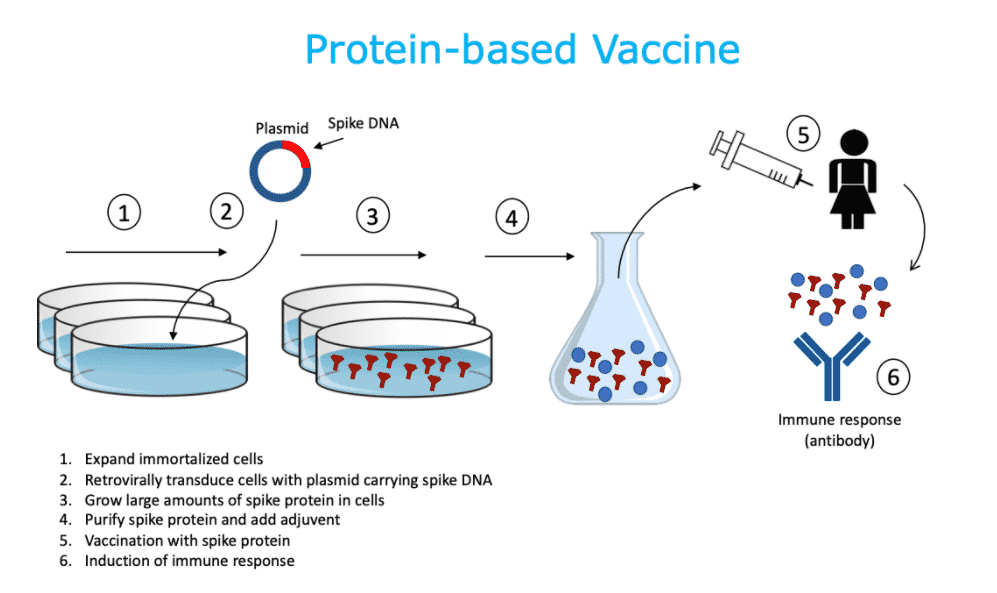
This vaccine candidate includes a part of the spike protein of SARS-CoV-2, known as the receptor-binding domain (RBD).
The studies were released in the Journal of Biological Chemistry and the PNAS.
6. HGCO19 – Gennova Biopharmaceuticals
| Developed By | Gennova Biopharmaceuticals |
| Vaccine Type | mRNA vaccine |
| Efficacy | N/A |
| Storage Temperature | 2-8°C |
| Dosage | – |
| Routes of administration | N/A |
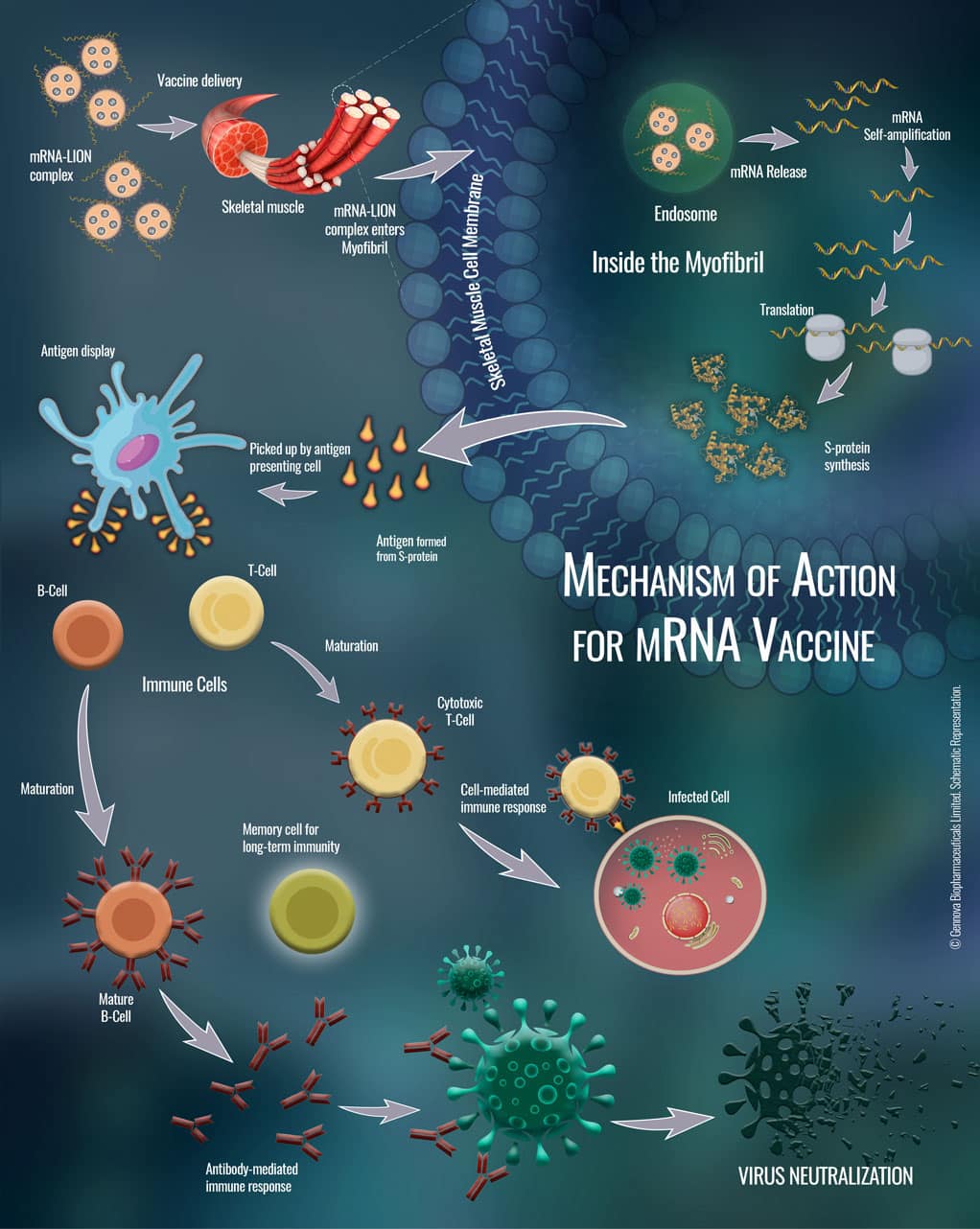
Gennova Biopharmaceuticals, in partnership with HDT Biotech Corporation, has actually collaborated to establish an mRNA vaccine. Their vaccine is referred to as HGCO19, has shown safety, immunogenicity, neutralization antibody activity in animal models.
mRNA vaccine candidate HGCO19 has a short, synthetic version encoding the spike protein of SARS-CoV-2. When the vaccine is injected into the body, the synthetic mRNA is taken to muscle cells, where it guides cells to make many copies of mRNA and copies of the antigen.
SARS-CoV-2 also utilizes its RNA genome led method to expand and express viral proteins inside the host cells. HGCO19 vaccine is also comparable and provides the benefit over other vaccine candidates to present the identically folded form of the spike protein of SARS-CoV-2 as it is described during its infection cycle.
7. Sputnik V
| Developed By | Gamaleya Research Institute of Epidemiology and Microbiology, Russia. (Approved to be used in India) |
| Vaccine Type | Non-replicating viral vector (adenovirus) |
| Efficacy | 91.4% |
| Storage Temperature | −18 °C |
| Dosage | Two dose |
| Routes of administration | Intramuscular injection |
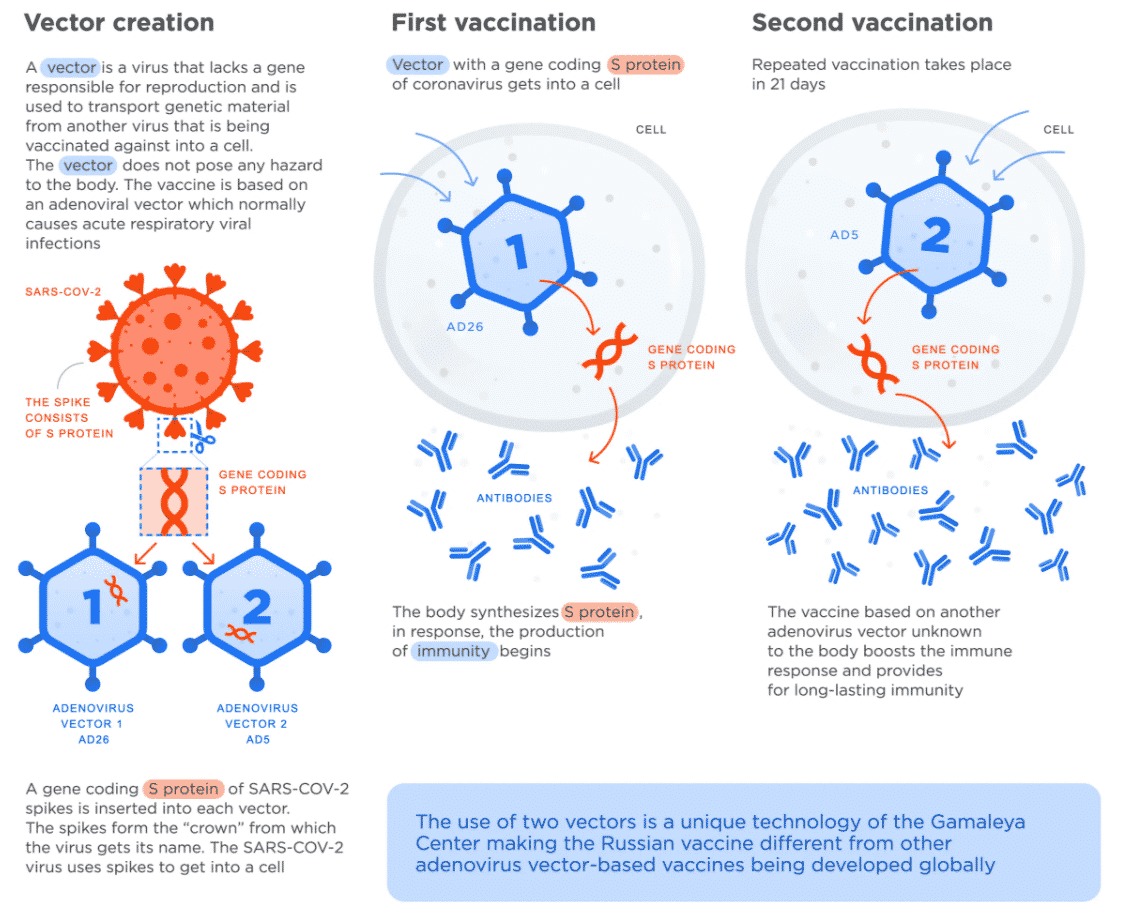
Sputnik V is the globe’s first registered COVID-19 vaccine based on a viral two-vector vaccine based on two human adenoviruses. The vaccine is manufactured in Russia but is approved for use in India and is being imported. Sputnik V is also known as Gam-COVID-Vac. It is progressing to the end of its clinical trials and soon will begin mass production. Sputnik V vaccine is titled after the 1st Soviet space satellite.
Clinical trials of Sputnik V have been introduced in India, Venezuela, Belarus, and the UAE, and RDIF’s global partners in India, Brazil, China, South Korea, and other countries will manufacture the vaccine for the international market.
This vaccine will cost less than $10 per dosage. The lyophilized form of the vaccine can be stored at +2 to +8 °C.
Two vector vaccine
Keyword: Coronavirus vaccines manufactured in India; Coronavirus vaccines and their types manufactured in India