Last Friday, Bharat Biotech stated that it will commence the Phase I Clinical Trial for its new intranasal coronavirus vaccine, BB154 by February or March of 2021. This new project was released a few days after the Hyderabad-centred company obtained the DCGI’s (Drugs Controller General of India) permission for India’s first indigenous vaccine against SARS-CoV2, the Covaxin. Along with Bharat Biotech’s Covaxin, Covishield produced by Serum Institute in collaboration with Oxford University and AstraZeneca also received permission for emergency use.
Bharat Biotech has revealed, in September 2020, that it has joined hands alongside Washington University School of Medicine (St. Louis) to develop the novel intranasal COVID-19 vaccine BB154. For Phase I Human Trials, they have planned studies at Vaccine & Treatment Evaluation Unit, St. Lous University, and India. So here is what we are aware of till now on Bharat Biotech’s intranasal COVID-19 vaccine.
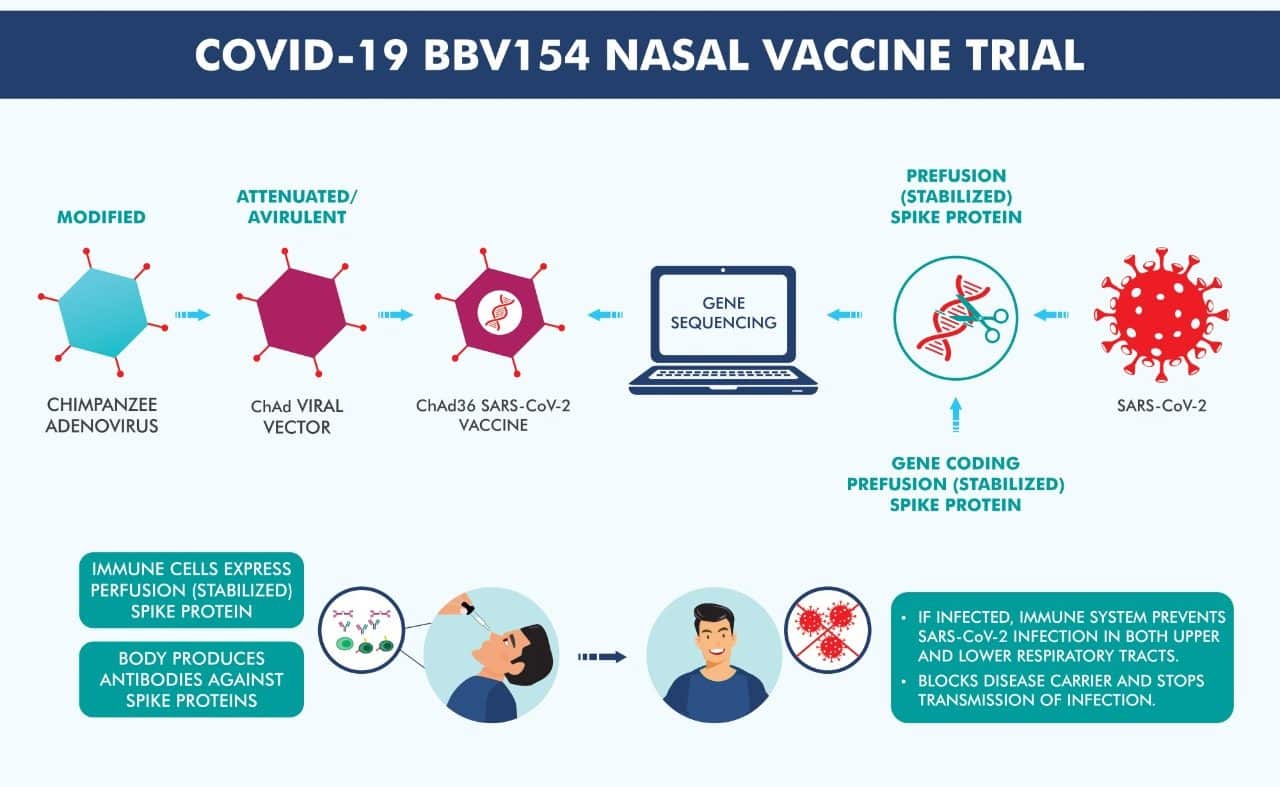
BBV154 to be a single-dose vaccine
Bharat Biotech’s intranasal vaccine, BBV154 is a novel adenovirus vector-based vaccine and is a single-dose vaccine against coronavirus. Based on the reports from the firm, a typical intranasal vaccine triggers an extensive immune response at the administration site, inside the nasal mucosa, which is important to impede the
COVID-19 infection and spread.Needle-free & non-invasive
One more benefit of an intranasal injection is that it is easy to administer and use- the vaccine is administered into the nose for immunization and doesn’t need a trained medical care worker or needle to inject the vaccine. Thus, this adds to the prevention of needle-related risks like infections and injuries. Additionally, an intranasal vaccine is an ideal vaccine for adults and children.
Dr. Krishna Ella, Bharat Biotech’s MD & Chairman claimed that apart from being convenient to use, an intranasal vaccine will lower the utilization of medical consumables like syringes, needles, and so on, profoundly affecting the total estimates for vaccination dive.
He also added that only one drop of the vaccine is needed for each nostril.
Demonstrates protective effectiveness
The firm reported that its new COVID-19 intranasal vaccine has proven exceptionally safe in rodent studies. The study findings, data, and techniques have been issued in an editorial section in Nature and in the renowned journal Cell.
The Company expressed that hamsters and rodents administered with a single dosage of ChAd-SARS-CoV-2-S demonstrated outstanding protection against the novel coronavirus, compared to a single or double administration of the same dose and vaccine intramuscularly. Besides the safeguarding effect, this vaccine also resulted in viral clearance in both upper and lower airways. Hence, the intranasal administration of ChAd-SARS-CoV-2-S can effectuate an immune system response in the nasal region, safeguarding against COVID-19 spread, infection, and disease.
On the other hand, Bharat Biotech has effectively finished the registration of 25,800 participants for the significant, Phase III Trials of indigenously developed Covaxin in association with ICMR (Indian Council of Medical Research) & NIV (National Institue of Virology). According to the vaccine developer, Covaxin is an inactivated, highly purified, and double-dose vaccine against SARS-CoV2, produced in a Vero cell production system with a superior performance history for safety studies of over 300 million doses.






























