COVID-19 Vaccine Using Rhabdovirus Vector – Funded By DBT
The global response to the unprecedented COVID-19 pandemic is drawing on the lessons learned from other disease outbreaks in the past.
The development of a COVID-19 vaccine must balance manufacturing speed and technical feasibility with clinical safety and immunogenicity trial outcomes, in the setting of a public health emergency. To reach the maximum number of people in the shortest possible period, the delivery must be simple and the manufacturing must be scalable.
Considering all these requirements, an agreement to develop a COVID-19 a vaccine based on an inactivated Rabies virus platform has been signed by Bharat Biotech International Ltd (BBIL), Hyderabad, and Thomas Jefferson University, USA. For this joint project, under the DBTBIRAC COVID-19 Research Consortia Initiative, the funding is provided by the Department of Biotechnology’s National Biopharma Mission.
A Rhabdovirus based vector that contains the S1 fragment of SARS-CoV-2 / COVID-19 spike glycoprotein has been developed by Thomas Jefferson University. Using betapropiolactone, the recombinant rabies virus harboring SARS-CoV-2 S1 fragment will be further inactivated.
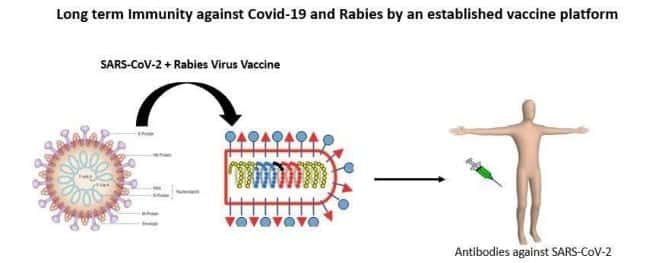
It is not new to use a Rhabdovirus vector for producing a vaccine. For many human vaccines, it has been used successfully. For example
, chemically inactivated Rabies Virus (RABV) vaccines are administered very widely to humans and are quite safe. In general, the codon-optimized foreign genes have been found to be highly expressed and are stable when introduced between RABV N and P genes. To develop several vaccines against pandemic and epidemic causing viruses like coronaviruses like SARS, MERS-CoV, Nipah virus, and Ebola virus, and more, the same platform is being used. Across diverse populations, the rabies vaccine has decades of safe use. Animal models based on challenge studies with the related MERS-CoV in two mouse models and alpacas (camelid) were protected to demonstrate a proof of concept. Since the RABV vaccine often provides life-long protection, long-term protection is expected.The complete development and commercialization of the vaccine into world markets will be taken up the world’s largest Rabies vaccine manufacturer, Bharat Biotech International Ltd (BBIL). For this vaccine, under the BIRAC/DBT Scheme from the Govt. of India, its vaccine facility has already received a Project Grant.
So far, BBIL has market authorization in over 65 countries and has commercialized 16 vaccines. It is the major supplier of the Oral polio vaccines (BIOPOLIO®) and the Rotavirus (ROTAVAC®) to the World’s Expanded Program of Immunization (EPI) and India. Globally, its products are being supplied to more than 115 countries and are registered in 33 countries. The company has a track record of low-priced vaccines at a high supply.
Source
COVID-19 Vaccine Using Rhabdovirus Vector






























