Transporter Protein Could Help Pump (Out) Bacteria with Antibiotics
The cell membrane is one of the great multi-taskers of biology. It provides structure for the cell, protects cytosolic contents from the environment, and allows cells to act as specialized units. A membrane is the cell’s interface with the rest of the world – it’s gatekeeper if you will. This phospholipid bilayer determines what molecules can move into or out of the cell, and so is in large part responsible for maintaining the delicate homeostasis of each cell.
Cells must bring in and remove different materials to survive. To accomplish this, they utilize different transporter proteins in their cell membranes, most of which a
re powered by what is called the proton motive force. The proton motive force is directed toward the inside of the cell in bacteria, which means that protons naturally want to move into the cell from the outside and do so if there is a pathway for them. These transporters allow the measured movement of protons into the cell — and in exchange for protons moving in, drug molecules get expelled.And encashing on this very phenomenon, scientists at the University of Wisconsin–Madison Department of Biochemistry have discovered that a cellular pump known to move drugs like antibiotics out of E. coli bacteria has the potential to bring them in as well, opening new lines of research into combating the bacteria.
UW–Madison biochemistry professor Katherine Henzler-Wildman and collaborators at the Washington University School of Medicine in St. Louis have now found that for E. coli’s small multidrug resistance transporter, called EmrE, proton, and drug movements are not as strictly coupled.
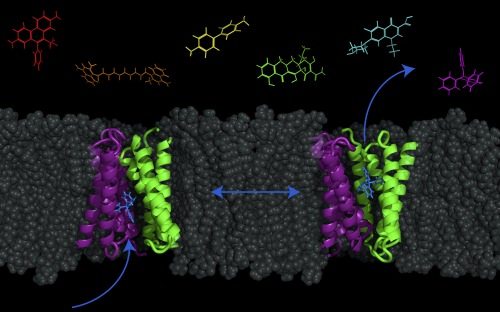
They have elucidated that this transporter can actually also move drugs and protons across the membrane in the same direction, as well as the opposite direction — introducing the option of moving molecules both into or out of the cell.
“The long-term implications are that this multi-drug transporter is reversible,” Henzler-Wildman says. “So instead of pumping drugs out to confer resistance, you have the possibility that you could use it to pump drugs in to kill bacteria. Drug entry is a big problem, so this is a new area to explore.”
“We started with a very basic science question of ‘how do these transporters work?’ and have stumbled upon this really translational direction,” she says. “People have been trying to target these kinds of pumps to stop antibiotic resistance to make antibiotics that we already have effective again. This suggests that you might be able to not just stop it but actually use these pumps to drive drugs into the cell as a new drug entry mechanism.”

Although found in many bacteria, this particular transporter has managed to be functionally elusive. While it does pump out antibiotics, it is not the main transporter that aids E. coli in antibiotic resistance, and it’s possible it has other purposes still undiscovered. They have only found that is transports a large number of molecules from dyes to antibiotics.
Traditionally, the model used to describe this transporter was the “pure-exchange model,” which required the strict, regimented movement of protons and the drug in opposite directions. However, Henzler-Wildman is now proposing a new model called the “free-exchange model,” where the combinations and direction of transport are much more flexible with many more options than previously thought.
“Having to rework the model and essentially rewrite the textbook on what we knew about the transporters will really change the way we think,” she says. “I’m actually going to teach this paper in our intro graduate course because it’s such a good story of how having a model in your head can limit your thinking and experiments and you really miss important things.”
The new model used magnetic resonance data to visualize these specific and previously unknown movements of the transporter. The scientists then studied how exactly the transporter responds in the test tube when, for example, it’s exposed to antibiotics, in order to confirm it works the way the structures showed.