Beyond the Great Wall: China’s Silent Biotech Revolution Reshaping Global Pharmaceuticals
Dr. Tang Shuying, a Postdoctoral Research Scholar, works in a renowned biotechnology research laboratory in Beijing. She was working late at night, adjusting her advanced microscope, and witnessed the beautiful world of Microbes. Five years ago, she was studying at Stanford University, but returned to her homeland in China immediately after completing her education. She had a zeal and passion that real and impactful innovations could happen in her homeland. Across the country, Researchers like her are developing extraordinary innovations in China, aiding the Country to become one of the leading nations in Biotechnology innovation. China’s biotechnology industry is setting global biotechnology trends and is becoming a guiding light to the world for Biotechnological innovations and development.
China was earlier known for developing cost-effective and low-cost Generic drugs, but now, in the present, it is developing innovative Therapeutics that are set to give tough competition to the European and American nations. This revolution in China is fueled by world-class talent, a leader mindset, as well as bold reforms, and is emerging as a Pharmaceutical powerhouse.
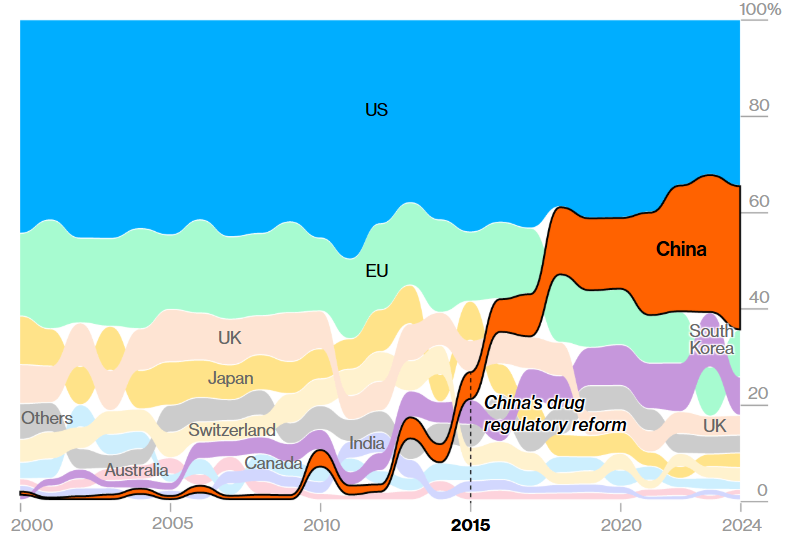
According to a Bloomberg News analysis, based on data from Pharma intelligence provider Norstella, in China, more than 1,250 innovative and new drugs, including those for weight loss, cancer, and other conditions, entered the development stage in 2024. This figure surpasses the statistics of the European Union and comes remarkably close to the United States’ count of 1,440, underscoring China’s rapidly expanding footprint in pharmaceutical innovation. China is not just catching up, it’s setting the pace.
Helen Chen, the managing partner at LEK Consulting in Shanghai, stated, “The scale itself is not something we’ve seen before.” She further added that, “The products are here, they’re attractive and they’re fast.”
Figure: The Annual share of various countries of innovative drugs entering development. Image Source: Norstella data analyzed by Bloomberg
China’s Biotech innovation Drug Development – From Reform to Reinvention
The scale of China’s growth stems from a comprehensive drug regulatory system implemented in 2015, when the country contributed only 160 innovative compounds, accounting for less than 6% of the global drug development pipeline. By comparison, it trailed significantly behind Japan and the United Kingdom.
These reforms were both regulatory and strategic in nature. China’s drug approval system underwent an overhaul to streamline the review process, enforce international standards for clinical data quality, and enhance transparency. Meanwhile, the government’s “Made in China 2025” initiative placed Biotechnology among its top ten strategic sectors, triggering a wave of public and private investment.
As a result, a new generation of companies — often founded by foreign-educated scientists and entrepreneurs — began populating China’s innovation ecosystem.
Daniel Chancellor, Vice President of Thought Leadership at Norstella stated, “Not only is it now almost at parity with the US, but it has that growth trajectory, it wouldn’t be sensationalist to suggest that China will overtake the US in the next few years purely in terms of numbers of drugs that it’s bringing through into its pipeline.”
Innovation Quality: A Leap Beyond Generics
China’s Biotechnology transformation isn’t merely quantitative, but it’s qualitative. Once known for imitation, Chinese Biopharmaceutical companies are now receiving global recognition from top Regulatory Agencies, including the US FDA (Food and Drug Administration) and the EMA (European Medicines Agency).
In the year 2024, China surpassed the EU in receiving expedited regulatory designations, including priority review, fast track, as well as innovative therapy, the indicators reserved for medicines showing significant promise in severe conditions.
One famous example is the CAR-T cell therapy developed by “Legend Biotech Corp,” in China, which has shown superior efficacy in treating certain Blood Cancers. It was co-developed with “Johnson & Johnson,” and earned several expedited designations and is considered clinically superior to a comparable US-developed CAR-T therapy.
China’s Biotech innovation Drug Development – Global Deals Signal Confidence in Chinese Science
Multinational Pharmaceutical companies are backing Chinese Biotechnology with record-breaking investments. In 2022, Summit Therapeutics paid $500 million upfront for the US rights to a novel Immunotherapy from “Akeso Inc.,” which demonstrated greater efficacy than “Merck’s Keytruda” in a Chinese Clinical Trial. The result was linked to China’s “DeepSeek moment,” a Biotechnology parallel to the country’s AI discoveries.
Later, in May 2025, “Pfizer Inc.” inked a $1.2 billion upfront agreement with 3SBio Inc. for a similar cancer drug — the largest initial payment ever made for a China-originated therapy.
According to a biopharma deal database, DealForma, these high-value licensing and co-development agreements are not only growing in size but also in frequency.
Speed, Scale, and Cost Advantage
China’s Biotechnology strength also lies in its operational efficiency. Thanks to a centralized Healthcare infrastructure and a vast patient pool, Chinese hospitals can complete Clinical Trial enrollment in half the time compared to their US counterparts. This is especially evident in early-stage trials for cancer and Metabolic Disorders such as Obesity.
Moreover, lower costs allow Chinese companies to run multiple trials in parallel, a risk-hedging strategy that’s often unaffordable elsewhere.
Andy Liu, China head at Novotech Health Holdings, a global clinical research organization, said, “They can leapfrog competitors in other countries.”
According to GlobalData, China has led the world in Clinical Trial initiations since 2021, further solidifying its position as a leader in early-stage Research.
China’s Biotech innovation Drug Development – Legacy Giants Reinvent Themselves
This wave of innovation isn’t confined to startups. Legacy Pharmaceutical companies such as “Jiangsu Hengrui Pharmaceuticals Co.,” once a leading Generic Drugmaker, have pivoted aggressively toward research and development.
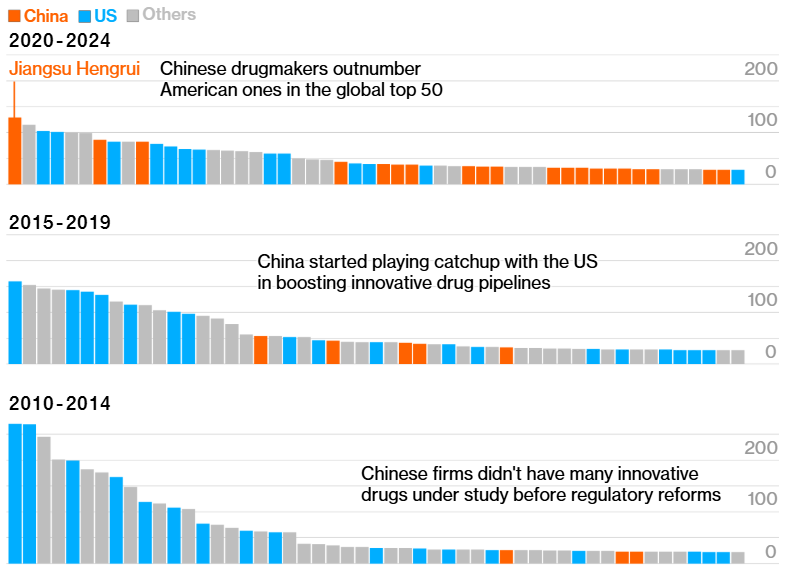
Between 2020 and 2024, Hengrui became the top global Pharmaceutical company for adding novel, innovative drugs to the global pipeline. Of the 50 companies with the highest number of novel drug candidates during this period, 20 companies were Chinese, a dramatic rise from just five in the preceding five years.
As China’s Biopharmaceutical industry gains ground, US policymakers have become increasingly concerned about the national security implications. A recent congressional commission warned that the US risks ceding its leadership in yet another critical Technical domain, one that could be vital not only for economic security, but for public Health in future Geopolitical crises.
Jack Burnham, analyst at the Foundation for Defense of Democracies, stated, “Biotech is at the forefront of the U.S.-China tech rivalry.”
Fears that reliance on Chinese-developed Medicines could one day be “weaponized” have prompted the US to call for investment screening, Regulatory reform, as well as export controls to boost American competitiveness.
In response, U.S. Health Secretary Robert F. Kennedy recently launched the initiative “Make American Biotech Accelerate,” aimed at streamlining domestic trial processes and enhancing local innovation pipelines.
Figure: The Pharmaceutical companies with the maximum number of innovative drugs entering into development, globally. Image Source: Norstella data analyzed by Bloomberg. Note: The Chart includes all companies tied for 50th place.
Looking Ahead: The Next Global Leaders?
Chinese Biotechnology companies are pressing forward with global ambitions.
“The Pharma industry is the best industry in the world,” said Michelle Xia, CEO of Akeso Inc. “At the end of the day, what we do benefits patients in China, in the U.S., and all around the world.”
As cities like Shanghai, Beijing, and Shenzhen rapidly ascend as Biotechnology hubs, they are increasingly viewed alongside legacy centers including Boston, Basel, and San Francisco.
China’s evolution from a Generic drug producer to a global innovation powerhouse marks one of the most dramatic shifts in modern Biomedical Science. Fueled by Policy reform, international talent, cost advantages, and substantial private-sector investment, China is not just closing the gap; it’s redefining it.
As China rises in the global drug innovation, the narrative is no longer about catching up; it’s about leading differently. This shift is not just revolutionizing development pipelines or profit charts; it’s redrawing the very geography of hope, healing, and human progress. The world must now look beyond the traditional strongholds of innovation in leading foreign countries and recognize that the next medical miracle could emerge from anywhere.
The coming years will determine whether this momentum leads to global dominance. Whether you’re a policymaker, a researcher, or a global citizen, this is the time to engage with the future of medicine. And the future is rapidly evolving beyond familiar borders!