Clinical Data Management Course for Beginners: What You Need to Know
When Srinidhi completed her degree in Biotechnology, she wanted to build a career in clinical research. She eagerly applied for multiple positions, only to face rejection, one after another. The reason? Every employer sought experience in Clinical Data Management (CDM), which was not covered in her regular college coursework.
However, Srinidhi was also deeply interested in the applications of Artificial Intelligence (AI) and Machine Learning (ML) in the field of clinical research. Determined to enhance her skill set, she enrolled in Biotecnika’s AI & ML in Biology program. As she progressed, she realized the importance of data integrity and management in clinical research, which led her to pursue a specialized Hands-on CDM course at Biotecnika.
By combining AI & ML expertise with CDM knowledge, Srinidhi became a strong candidate for interdisciplinary roles. Today, she is working as a Junior Clinical Data Analyst at an MNC, utilizing AI-driven tools for data management and analysis. Her journey proves that perseverance, as well as the proper training, can turn setbacks into success, opening doors to high-growth careers in clinical research and healthcare technology.
This article explores what a Clinical Data Management course
for beginners entails, why it’s essential, and how Biotechnology platforms like Biotecnika and others can help you kickstart your journey.Table of Contents
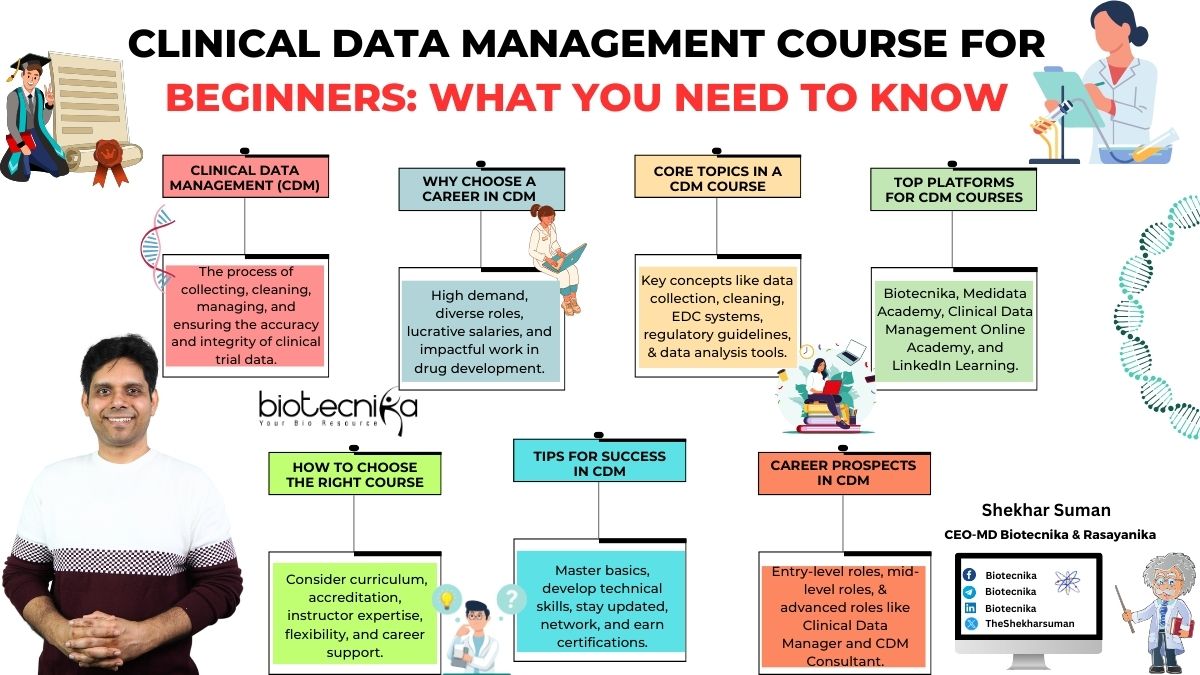
What is Clinical Data Management (CDM)?
Clinical Data Management (CDM) is a crucial part of Clinical Research that focuses on collecting, cleaning, and managing data from clinical trials. It ensures that the data is accurate, reliable, and statistically sound. This enables pharma companies to make informed decisions about drug safety and efficacy.
Key responsibilities in CDM include:
- Data Collection: This involves designing user-friendly data entry forms and ensuring accuracy from the entry point to integrating data from various sources like laboratory reports, patient records, and clinical assessments. An Electronic Data Capture (EDC) system is used to collect data.
- Data Cleaning: The process includes reconciling discrepancies, running validation checks, and ensuring adherence to predefined quality standards while reducing errors.
- Database Management: Choosing the appropriate database management system, maintaining data security, and ensuring compatibility with statistical analysis tools.
- Regulatory Compliance: Ensuring adherence to global standards such as FDA (Food and Drug Administration) and GCP (Good Clinical Practice) regulations. This involves maintaining thorough documentation, understanding regulatory requirements, and preparing for inspections and audits.
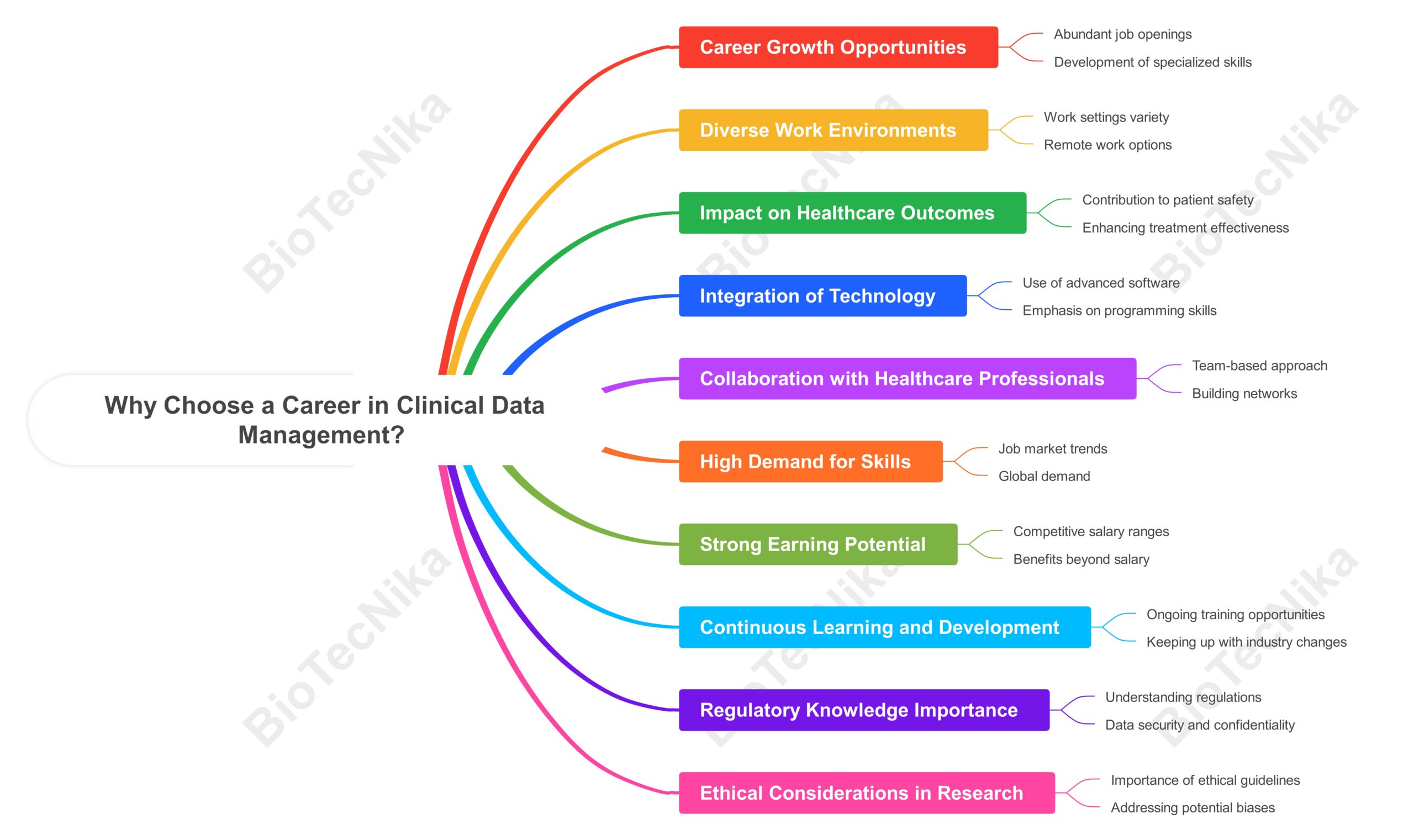
Why Choose a Career in CDM?
- High Demand: With advancements in drug discovery and personalized medicine, the demand for skilled professionals in CDM is increasing.
- Diverse Opportunities: Each role offers unique challenges and specialization in different areas of clinical research, ranging from Clinical Data Coordinators and Managers to Database Specialists.
- Impactful Work: Your contributions directly influence drug development and patient safety. Knowing that your work helps bring life-saving treatments to market can be highly fulfilling.
What to Expect in a Clinical Data Management Course for Beginners?
Core Topics Covered
A foundational CDM course introduces you to the key concepts and tools of the field. Typical modules include:
- Introduction to Clinical Research: Overview of the drug development process, including preclinical studies and clinical trial phases (Phase I to IV), and the role of CDM in ensuring trial success.
- Clinical Data Management Basics: Learn the fundamentals of data collection, cleaning, and validation. Understand the lifecycle of clinical trial data and the importance of maintaining data integrity.
- Electronic Data Capture (EDC) Systems: Training with tools like Medidata Rave, Oracle Clinical, or OpenClinica.
- Regulatory Guidelines: Thorough understanding of Good Clinical Practice (GCP), FDA regulations, and International Council for Harmonisation (ICH) guidelines.
- Data Analysis Tools: Learning tools like SAS and SPSS to analyze clinical trial data and generate reports.
Hands-On Training
Most courses provide practical training, allowing learners to:
- Create and manage clinical databases using industry-standard tools.
- Perform data validation and cleaning to ensure high-quality datasets.
- Generate clinical data reports for regulatory submissions and internal reviews.
Certification
After course completion, they offer certificates that will boost your resume and showcase your expertise to hiring managers. These certifications are often recognized globally and add value to your resume.
Top Platforms Offering Clinical Data Management Courses
1. Biotecnika
One of the most trusted names in life science education, offering industry-relevant courses tailored for beginners.
Course Features:
- A comprehensive curriculum covering the basics of CDM and EDC systems.
- Live sessions with industry experts provide real-world insights and practical knowledge.
- Hands-on projects and assignments designed to simulate actual CDM tasks.
- Affordable pricing and flexible schedules for students and working professionals.
- Job placement assistance and career counseling for Biotecnika students.
- Interactive learning with quizzes, case studies, and discussions.
- A platform offering career advice, exam alerts, scholarships, research proposals, conferences, workshops, internships, professional networking, and certifications.
Whatsapp an Expert for more details
2. Medidata Academy
A leading academy providing cloud-based solutions for clinical trials, with specialized training in Medidata Rave, a widely used EDC system.
Course Offers:
- Specialized training in Medidata Rave.
- Access to expert mentors and pharmaceutical industry exposure.
3. Online Platforms for CDM
Various online platforms provide CDM courses for beginners, offering both theoretical knowledge and practical skills.
Key Factors:
- Exposure to real-world clinical data management work.
- Insights into regulatory compliance, database management, and data analysis.
4. LinkedIn Learning
LinkedIn Learning offers short, beginner-friendly courses on CDM and life science topics.
Notable Features:
- Small course structure for students with busy schedules.
- Integration with LinkedIn profiles for showcasing certificates and gaining recruiter visibility.
How to Choose the Right Course?
When selecting a CDM course, consider:
- Course Curriculum: Ensure the course covers both practical and theoretical aspects of CDM, such as regulatory guidelines and EDC systems.
- Accreditation: Select courses recognized by industry bodies, institutions, or universities to enhance career credentials.
- Instructor Expertise: Look for courses taught by experienced CDM professionals with real-world insights.
- Flexibility: Choose programs that fit your learning pace and schedule.
- Career Support: Opt for platforms offering placement assistance, resume-building workshops, and career counseling.
Tips for Success in Clinical Data Management
- Master the Basics: Gain knowledge of core concepts such as database design, data validation, and regulatory compliance.
- Develop Technical Skills: Gain proficiency in EDC systems such as Medidata Rave and statistical tools like SAS.
- Stay Updated: Keep up with emerging tools, industry trends, and evolving regulations.
- Network: Participate in webinars, workshops, and industry events to build connections.
- Earn Certifications: Enhance your resume with certifications from recognized industry platforms.
Starting a career in any field may seem daunting, but with the right guidance, training, determination, and dedication, anyone can succeed. Platforms such as Biotecnika and others offer comprehensive courses that boost the skills needed to thrive in this growing field.
Remember, every expert was once a beginner. Take the first step, invest in your future, and open doors to a rewarding career in CDM where your work can make a real difference in healthcare. Though the journey may be challenging, the rewards of a career in CDM are truly life-changing!








































The course is very good
Yes, it’s very good and working course.
I have a question…
Can I do CDM course after BMLT?