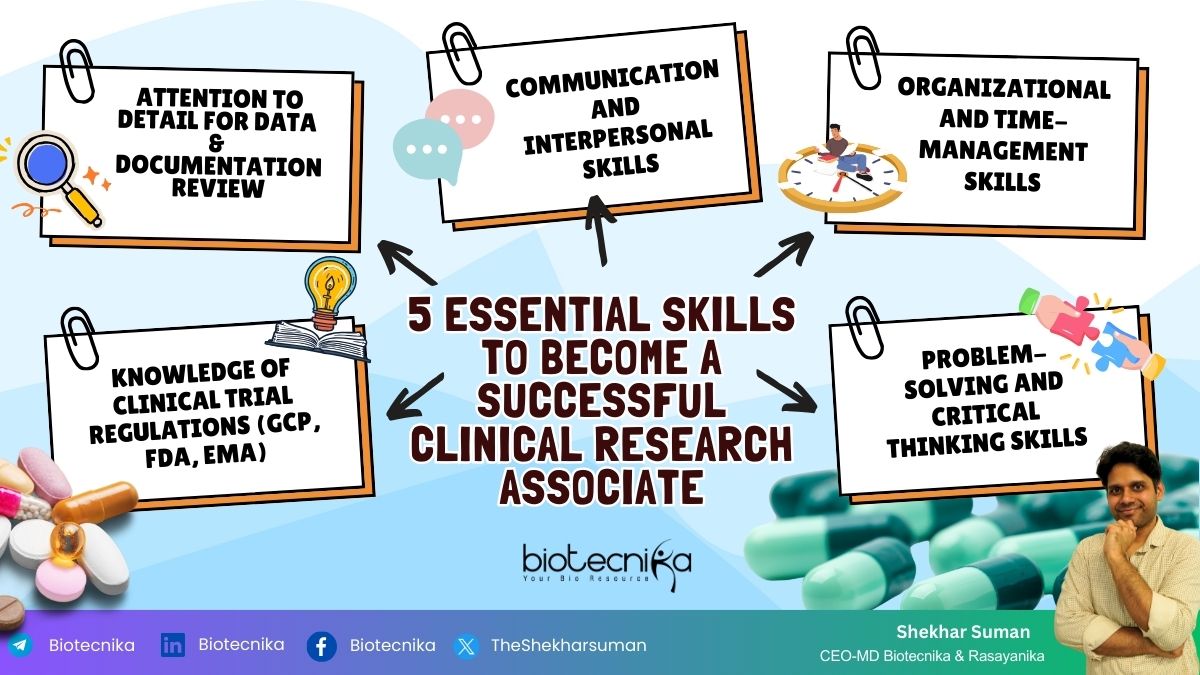
Clinical Research Associate Career – 5 Essential Skills Needed to Become a Successful Clinical Research Associate
According to recent data, the clinical trial industry was valued at USD 80.7 billion in 2023 all across the world and is projected to reach USD 123.5 billion by 2030. The domain is expected to grow at a compound annual growth rate (CAGR) of 6.3% by 2030, indicating a steady increase in demand for clinical research associates (CRAs).
To succeed in the field of clinical research and to become a successful CRA, it is important to develop a diverse set of skills that include technical expertise, organizational abilities, and interpersonal acumen.
If you also aspire to be a clinical research associate and explore the dynamic field od clinical research, then you are in the correct place.
This article will take you through the five essential skills that are required to excel as a CRA and help you take a few more steps toward your dream job,
Table of Contents
1. Comprehensive Knowledge of Clinical Research and Regulations:
To be a successful CRA, it’s primary to have a deep understanding of the clinical research landscape, including ethical considerations, scientific principles, and regulatory frameworks. The knowledge of clinical research forms the primary foundation to make sure that the trials are conducted in a manner that upholds participant safety and maintains the integrity of the data.
Why is it Important?
- Ethical Oversight: In every clinical trial, participant safety is of the highest priority, and informed consent is required. A CRA ensures the patients understand the risks and benefits of participating in a study and that ethical guidelines are followed diligently.
- Regulatory Compliance: Each country has its own set of regulations for governing clinical trials. For example, the FDA oversees clinical trials in the United States, while EMA governs Europe. A CRA makes sure that the trials align with these regulations to avoid penalties or invalidation of data.
- Scientific Rigor: Understanding the science behind the trial helps a CRA monitor whether the protocol is being followed as designed, which directly impacts the validity of the results.
Key Responsibilities of a Clinical Research Associate:
- Ensuring Protocol Compliance: During site visits, a CRA verifies that the site staff strictly adheres to the trial protocols. Any deviation from the protocols can be responsible for compromising the study.
- Audits and Inspections: A CRA prepares sites for regulatory audits by making sure that all the documentation, processes, and data meet the required standards.
- Keeping Up with Changes: Clinical Trials Regulations keep changing frequently. It is essential for CRAs to stay up-to-date about new GCP guidelines or changes in adverse event reporting requirements.
Development Strategies:
- Obtain certifications like GCP, offered by SOCRA or ACRP. These programs provide in-depth knowledge about clinical trial ethics and regulation.
- Attending global conferences like DIA or specialized workshops focusing on specific therapeutic areas or emerging trends in clinical research can help develop your skills as a CRA.
- Gain exposure by assisting in real-world clinical trials, which allows you to apply theoretical knowledge to practical situations.
Clinical Research Training Program With Applied Online Project Work – Starts 7th FEB 2025
2. Attention to Detail:
Clinical Research Associate Career
Clinical trials involve managing vast amounts of data, from patient records to regulatory documentation. Any error or discrepancy, no matter how small it is, can lead to incorrect results, regulatory delays, or even trial suspensions. CRAs must have a keen eye for detail to ensure the accuracy and completeness of every aspect of the trial.
Why is it Important?
- Data Integrity: Sponsors and regulatory authorities rely on accurate data to make critical decisions about the safety and efficacy of the treatments. Even minor errors can undermine the validity of a study.
- Patient Safety: It is the responsibility of a Clinical research associate to ensure accuracy in documenting adverse events or following dosing protocols directly impacting participant well-being.
- Regulatory Compliance: Missing or inconsistent data can result in regulatory findings or trial rejections.
Key Applications:
- Data Verification: Clinical Research Associates cross-check data entered into electronic data capture (EDC) systems against source documents, such as patient charts or lab reports.
- Identifying Discrepancies: Spotting inconsistencies in patient enrollment numbers, treatment regimens, or visit schedules ensures errors are corrected early.
- Audit Preparations: A CRA makes sure that all trial-related documents, including informed consent forms and monitoring reports, are accurate and complete for audits.
Development Strategies:
- Work on mock CRFs or participate in training that simulates real-world scenarios to improve your skills and have a better understanding of trials.
- Learn and implement software like Medidata or Veeva to streamline document reviews and track discrepancies.
- Joining quality assurance teams to learn common errors and how to prevent them.
3. Effective Communication and Interpersonal Skills:
A CRA is accountable for many aspects of leading a successful trial, one such aspect is constant interaction with various stakeholders, from site staff and principal investigators to sponsors and regulatory bodies. Effective communication helps in keeping everyone involved in the trial is on the same page, reducing misunderstandings and facilitating collaboration and resulting in a successful trial.
Why is it Important?
- Building Relationships: Successful trials are a result of a team effort. Forming good relationships with the site staff ensures smooth operations and quick resolution of issues.
- Conflict Resolution: During clinical trials, many disagreements may arise, such as discrepancies in protocol interpretation. A CRA’s communication skills are key to resolving these efficiently.
- Transparency: Sponsors rely on accurate and detailed updates about site performance and trial progress.
Key Applications:
- Training Site Staff: Clinical research associates often provide train the site teams on protocols, safety measures, and regulatory updates, and also make sure that everybody understands and performs their roles to the best of their ability.
- Resolving Issues: When challenges arise, such as a site struggling with patient recruitment, CRAs mediate discussions to identify and implement solutions.
- Report Writing: Its necessary that monitoring visit reports must be clear, concise, and comprehensive, which gives sponsors a clear idea about the site performance.
Development Strategies:
- Enhance your ability to draft clear and professional reports by taking technical writing courses.
- Practice scenarios where you address site staff concerns or present findings to sponsors.
- Attend workshops to improve confidence and clarity when presenting updates or training site teams.
4. Organizational and Time-Management Skills
Clinical Research Associate Career – CRAs have to manage multiple sites at the same time, and they juggle tight deadlines, and oversee a variety of tasks, from monitoring visits to data reviews. Being organized ensures that all responsibilities are met without compromising quality or timelines.
Why It’s Important:
- Efficient Monitoring: To conduct a successful and well-coordinated trials, CRAs must plan their site visits strategically and make sure they have enough time for adequate time for detailed inspection while meeting deadlines.
- Documentation Management: As the clinical trials progress, they generate a significant amount of paperwork alongside, and proper organization is crucial for easy retrieval and compliance.
- Avoiding Burnout: If CRAs have good time management skills, it helps them balance their workload, especially when they have to handle multiple sites at the same time.
Key Applications:
- CRAs must coordinate visits with site staff while making sure there is minimal disruption to trial activities.
- Balancing urgent issues, such as reporting serious adverse events, with routine tasks like data monitoring.
- Keeping accurate and updated records of trial progress, deviations, and corrective actions.
Development Strategies:
- Using project management tools like Asana or Monday.com to help keep track of tasks,
deadlines, and progress of the clinical trials.
- Learn methods like the Eisenhower Matrix to differentiate between urgent and important tasks.
- Adopt strategies to manage stress and maintain focus, such as regular breaks or time-blocking.
Get Trained in Clinical Research – From Basics To Advanced + Do Project Work + Gain Experience
5. Problem-solving and Critical Thinking Skills
As a Clinical research associate, professionals might encounter unexpected challenges, from patient recruitment issues to protocol deviations. During such times clinical research professionals need to think critically and act decisively to address these issues without making any compromises with the integrity and timeline of the clinical trial.
Why It’s Important:
- Ensuring Continuity: Prompt problem-solving prevents small issues from escalating into major disruptions.
- Maintaining Compliance: Finding solutions that align with the regulatory standards to ensure the trial remains valid.
- Optimizing Outcomes: Creative thinking is essential to improved recruitment strategies or more efficient monitoring processes.
Key Applications:
- Investigating Deviations: If a site fails to follow the protocol, it is the responsibility of a CRA to understand the cause and implement corrective actions.
- Addressing Recruitment Challenges: Collaborating with sites to refine patient outreach strategies or adjust inclusion criteria.
- Handling Emergencies: Managing unexpected events, such as adverse reactions or equipment failures, in a way that minimizes impact.
Development Strategies:
- Participate in case studies or simulations that mimic real-world challenges.
- Work alongside experienced CRAs or mentors to learn practical problem-solving approaches.
- Stay updated on industry innovations, such as AI-based tools for trial optimization, to tackle challenges more effectively.
To Begin your Clinical Research Associate Career, To become a successful Clinical Research Associate, a balance of technical expertise, organizational prowess, and interpersonal skills is required.
By increasing their expertise, learning all the essential skills outlined above, and embracing continuous learning, CRAs can excel in their roles, contributing to the success of clinical trials and the advancement of medical science.
These skills not only enhance professional growth but also play a crucial role in ensuring that life-changing treatments reach patients safely and efficiently.








































Good to be part of this team