CSIR UNIT 1B Notes PDF Download – CSIR NET Life Science Notes
Introduction to Proteins
Proteins mediate nearly every process that takes place inside a cell. They are the most abundant biological macromolecules in cells. All proteins, regardless of organism, are composed of the same set of 20 amino acids that are incorporated into them during translation. Due to the nearly limitless variety in the sequences of amino acids in proteins, nearly all imaginable functions can be encoded in proteins.
Amino Acids
Amino Acids are small organic molecules that play several important roles in living organisms: They are the principal building blocks of proteins, Nature’s most functionally diverse biomolecules. They serve as precursors for many biologically active molecules, such as neurotransmitters (e.g. dopamine, serotonin, GABA, epinephrine); local mediators (e.g. the allergy mediator histamine); energy-related metabolites (e.g. creatine, citrulline, carnitine); the oxygen-binding molecule ‘heme’; DNA bases called purines. Serve as an energy source during prolonged fasting, diabetes, when the diet is rich in proteins. Some act as regulators of gene expression and cellular signaling. This affects multiple physiological processes that are related to growth, maintenance, reproduction, and immunity.
CSIR UNIT 1B Notes
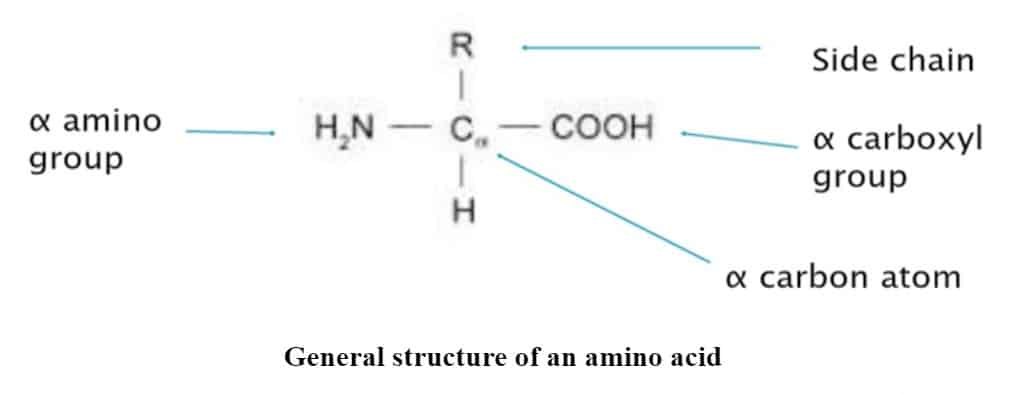
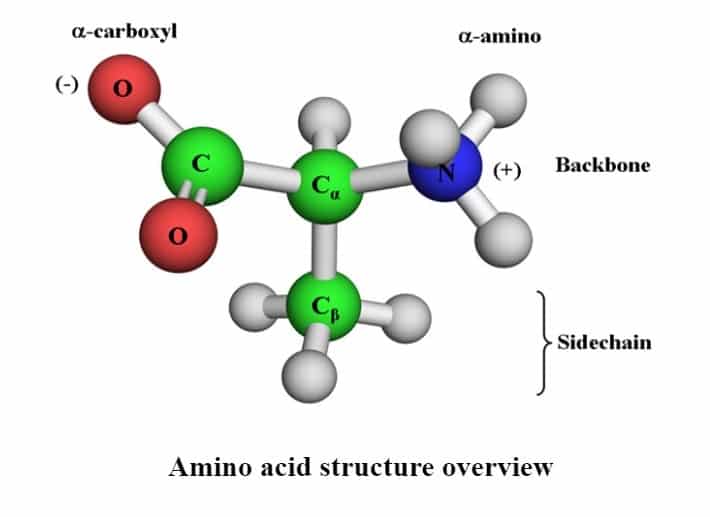
The general structure of an amino acid
Amino acids consist of a functional group/side chain R attached to an amine group (NH2) and a carboxyl group (COOH). Key elements of amino acids are C, H, O, and N. There might be other elements found in the side chains of certain amino acids.
All amino acids that appear in proteins (a.k.a. ‘α-amino acids) possess a structure that includes a central carbon atom called Cα, surrounded by four substituents: a hydrogen atom, an amino group (α-amino), a carboxyl group (α-carboxyl), and a fourth group referred to as side-chain. There are roughly 300 types of amino acids in nature, only 20 of which serve as building blocks of proteins. Others function as metabolites, messengers, and regulators of biological processes. There are two amino acids, selenocysteine and pyrrolysine, which are not considered as one of the 20 types of protein building blocks, although they are genetically incorporated into proteins. This is because the two amino acids appear only in certain proteins, whereas the other 20 appear in all. Referred to as the ’21st & 22nd amino acids in some books. Side Chains or R groups vary in structure, size & electric charge and hence influence the chemical nature of the amino acid. The average molecular weight of an amino acid is 110 daltons.
The α-carboxyl group has a low pKa (~2), and is therefore deprotonated and negatively charged at physiological pH (7). The α-amino group has a high pKa (9–10), and is therefore protonated and positively charged at physiological pH. The side-chain is chemically different in each of the amino acids. They determine the uniqueness of the 20 natural amino acids found in proteins. Amino acids in proteins almost exclusively possess an L configuration. Amino acids with D configuration can be found in microorganisms (e.g. in the bacterial cell wall and in antibiotic peptides) and in certain animals (e.g. the frog skin peptide ‘dermorphin’).
Stereochemistry of α–amino acids – CSIR UNIT 1B Notes
For all the amino acids except glycine, the α -carbon atom is bonded to four different functional groups: a carboxyl group, an amino group, a side chain ‘R’, and a hydrogen atom. α – carbon atom is, therefore, asymmetric in all a.a except glycine. The α- carbon atom is chiral i.e. it shows optical activity. Most amino acids have one chiral carbon atom and thus have two possible stereoisomers.
No. of stereoisomers = 2n, where ‘n’ is the no. of chiral carbons
Amino acids have two stereoisomers that are non-superimposable mirror images of each other and are called enantiomers.
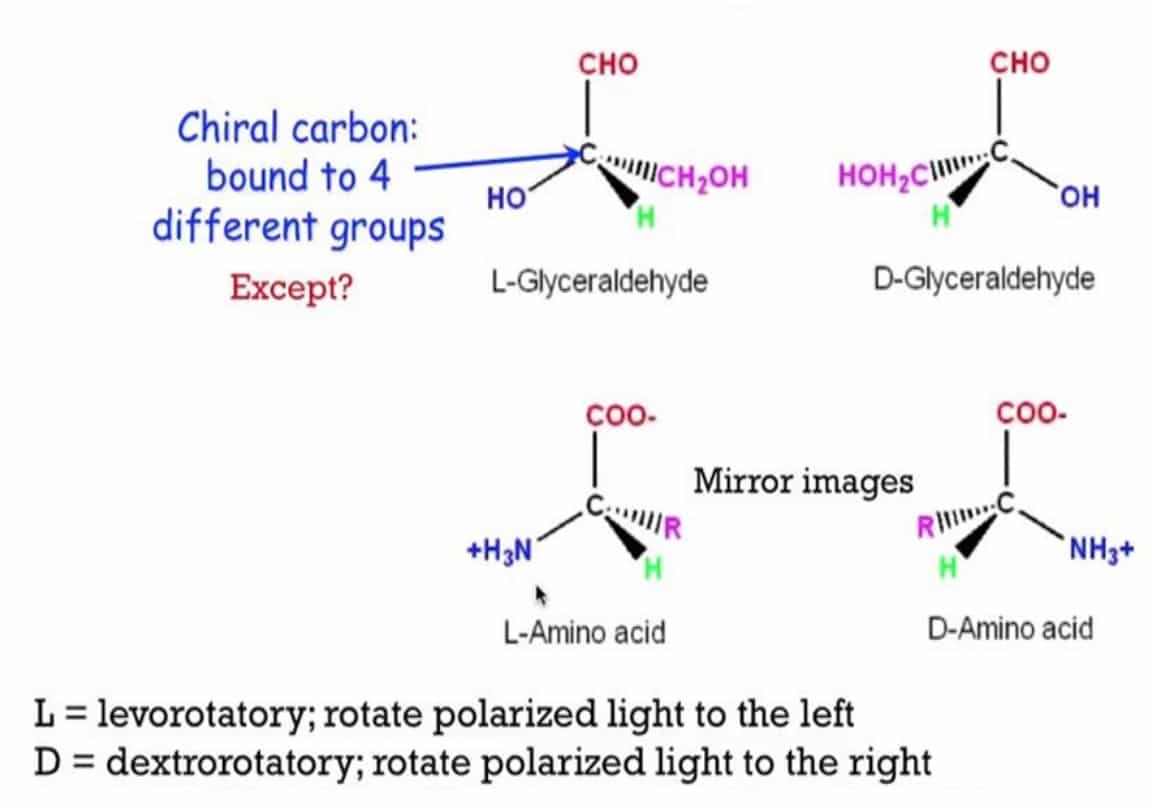
All amino acids found in proteins are L-enantiomers. Stereochemistry in amino acids plays a role in overall protein structure. D-amino acid residues have been found only in a few small peptides, including some peptides of bacterial cell walls and some peptides.
Amino acids can be classified by the R group
Amino acids can be grouped into five main classes based on the properties of the ‘R’ group, mainly their polarity.
- Non-polar aliphatic R groups: Alanine, Valine. Leucine, Isoleucine, Glycine, Methionine, and Proline
- Aromatic R groups: Phenylalanine, Tyrosine, and Tryptophan
- Polar Uncharged R groups: Serine, Threonine, Cysteine, Asparagine, and Glutamine
- Positively charged (Basic) R groups: Lysine, Arginine, and Histidine
- Negatively charged (Acidic) R groups: Aspartate and Glutamate
It is customary to group the 20 natural amino acids found in proteins into 4 types, according to the polarity of their side chains. Amino acids that have polar side chains are hydrophilic. That is, they tend to appear on the surface of water-soluble proteins where they can interact favorably with the surrounding water. Amino acids that have nonpolar side chains are hydrophobic. That is, they tend to be buried inside proteins, where they are away from water and can interact favorably with each other. CSIR UNIT 1B Notes.
Nonpolar, aliphatic R groups
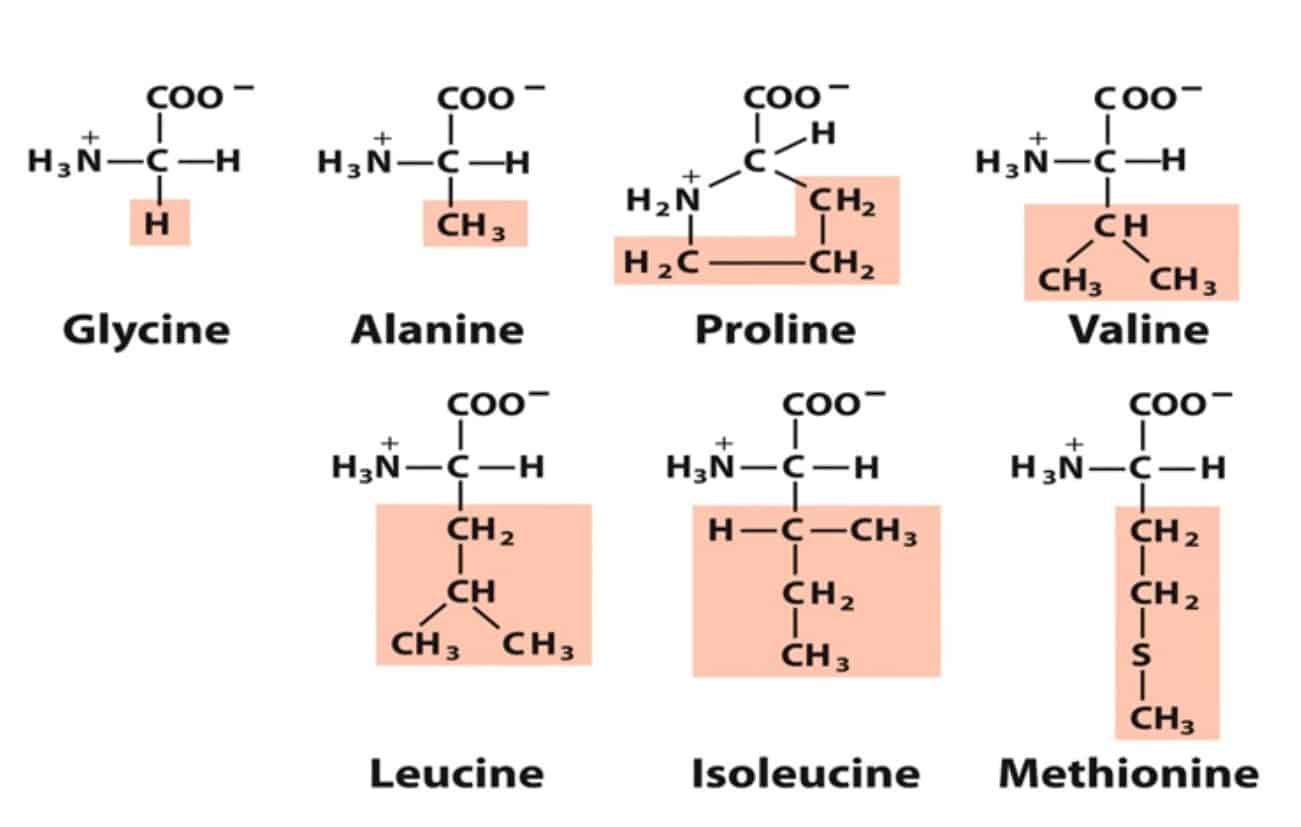
Alanine, Valine, Leucine, and Isoleucine tend to cluster together owing to hydrophobic interactions. Methionine contains a sulfur atom in its side chain. Proline has an aliphatic side chain with a distinctive cyclic structure. It has an amino group and hence reduces structural flexibility.
Download CSIR NET UNIT 1(A) Notes Here
Nonpolar, aliphatic R groups
Glycine (gly, G) has a single hydrogen atom as a side chain. Alanine (Ala, A) has a methyl group (CH3) as a side chain. Valine (val, V), leucine (leu, L) and isoleucine (ile, I) have a branched aliphatic side chain. Methionine (met, M) has a sulfur-containing linear aliphatic side chain. Proline (pro, P) has an aliphatic side chain that is covalently attached to the α-amino group. In water-soluble proteins, nonpolar amino acids reside mainly in the hydrophobic core. There, their interactions with each other (the ‘hydrophobic effect‘) is what hold together the 3-dimensional fold of the protein. In membrane-bound proteins, nonpolar amino acids reside on the surface, where they can interact favorably with membrane lipids. Finally, nonpolar interactions involving these amino acids serve as a driving force for the binding of ligands and substrates to the protein. Some interesting facts about nonpolar amino acids are as follows: Methionine, despite being overall nonpolar, is still able to interact weakly with polar species, such as metals. This is thanks to the nonbonding electrons of methionine’s sulfur atom, which are only weakly held by the nucleus. Glycine, having only hydrogen as a side chain, confers flexibility to the protein areas in which it is present. Proline confers rigidity due to its side chain being fused to the rest of the amino acid. The branched amino acids (valine, leucine, isoleucine) are an important source of energy in muscles and signal cells to synthesize proteins.
Phenylalanine (phe, F) has a phenyl group in its side chain. Tyrosine (tyr, Y) has a phenol group in its side chain. Tryptophan (trp, W) has an indole group in its side chain. Relatively nonpolar (hydrophobic), all participate in hydrophobic interactions. Hydroxyl groups of tyrosine can form H- bonds, important functional groups of many enzymes. Tyr & Trp are more polar than Phe because of the Tyr-OH group and nitrogen of the Trp indole ring. All amino acids absorb in the infrared region.
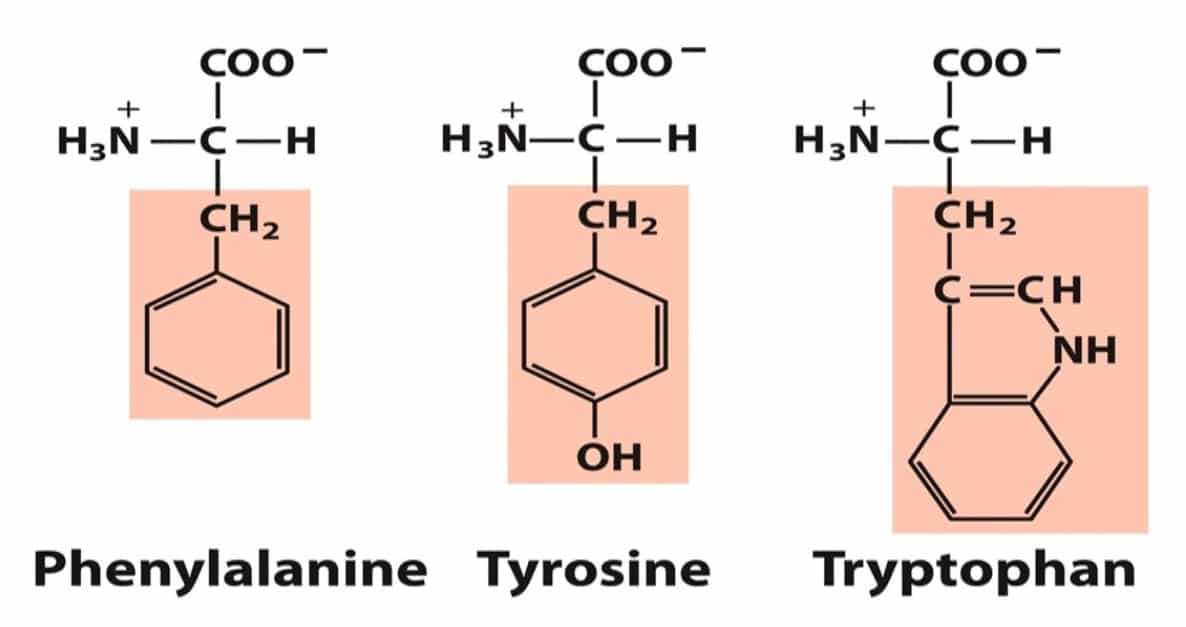
In contrast to the other amino acid groups, this one is considered not according to the polarity of the side chain but rather on its aromatic nature. Aromatic groups contain delocalized π electrons which can interact with similar electrons in other aromatic groups, as well as with positively-charged groups. CSIR UNIT 1B Notes
Indeed, all of these interactions are observed in proteins between aromatic side chains. The aromatic amino acids are important in forming closed scaffolds within proteins, especially binding sites for ligands and substrates. Here are interesting facts about some aromatic amino acids: Like serine and threonine, tyrosine may also become phosphorylated on proteins involved in cellular communications. A well-known example is the membrane-bound receptors which respond to growth factors. The binding of the latter to these receptors results in their phosphorylation, which in turn conveys the signal into the cell and results in cellular division. Genetic defects that allow for hormone-independent phosphorylation of these proteins often lead to cancer. The phenol group of tyrosine also participates in different mechanisms of enzymatic catalysis (e.g. nucleophilic attack, acid-base catalysis, and stabilization of reaction intermediates). The indole group in tryptophan’s side chain is capable of participating in different polar and nonpolar non-covalent interactions with other chemical species.
Therefore, it is common in protein binding sites. It also participates in enzymatic catalysis and electron transfer. Tryptophan’s side chain fluoresce when absorbing UV light. This allows biochemists to identify proteins or study changes in their structure by UV-irradiating them and then recording the fluorescence of their tryptophan amino acids. The side-chains of the aromatic amino acids, phenylalanine, tyrosine, and tryptophan, overall are very hydrophobic. The R group of tyrosine also contains a polar hydroxyl group that can participate in Hydrogen bonding interactions. The R groups of tyrosine, and particularly tryptophan, absorb ultraviolet light at a maximum of 280 nm wavelength. Light absorption by these amino acids is exploited in detecting and quantifying proteins in the laboratory using the technique of absorbance spectrometry. Tryptophan absorbs almost four times as much as Tyrosine. Phenylalanine contributes little to the spectroscopic property.
Polar, uncharged R groups
R-groups are more soluble in water (more hydrophilic and contain functional groups that form hydrogen bonds with water. Serine and Threonine have hydroxyl (OH) groups. Cysteine has a sulfhydryl/thiol group (-SH). Asparagine and Glutamine have amide groups. Asparagine and Glutamine are amide derivatives of Aspartate and Glutamate respectively. In proteins, polar-uncharged amino acids form hydrogen bonds with each other and with the protein’s ligand/substrate. This confers specificity to protein-ligand interactions, in contrast to the hydrophobic effect which confers stability and serves as a driving force for the interactions. Some of the polar-uncharged amino acids also function as nucleophiles in enzymatic catalysis. Serine and threonine are often phosphorylated in proteins that participate in cell-cell communication. CSIR UNIT 1B Notes.
This modification is reversible and serves as a chemical, signal transduction tag. The two amino acids may also function as nucleophiles in enzymatic catalysis, thanks to the hydroxyl group in their side chain. Serine and threonine on the surface of membrane/secreted proteins may also be glycosylated (attached with sugar groups). This protects the protein and increases its water solubility. In some cases, the attached sugar groups serve also as a recognition code for other extra-cellular elements. Cysteine, despite being polar, tends to appear in the core of water-soluble proteins. Under oxidizing conditions, its thiol side chain deprotonates and tends to form a (covalent) disulfide bond with a thiol group of a neighboring cysteine. These bonds are important for stabilizing proteins that are secreted from cells (hormones, antibodies, some enzymes).
Cysteine also serves as a nucleophile and an electron-transfer agent in enzymatic catalysis. Both are the result of the non-bonding electrons of cysteine’s sulfur atom, conferring it high chemical reactivity and the ability to exist in different oxidation states. The carboxamide group of glutamine and asparagine can serve as both hydrogen bond donor and acceptor. As a result, these amino acids are commonly involved in hydrogen bond networks within proteins. This group in asparagine is also glycosylated in membrane/secreted protein.
Disulphide Bond – Contd…
Fill Up the Form & Follow the procedure to download the complete FREE PDF of CSIR NET UNIT 1B Notes
Keyword: CSIR NET UNIT 1B Notes by Biotecnika, CSIR NET UNIT 1 Notes, CSIR NET Life science notes.










































Also keep provide chemical sciences notes