Biosafety & R&D – Important But Ignored Aspect
Hello every one, here’s presenting the 1st place winner of the Biotecnika Article Writing Contest – Ms. Sulagna Roy! Read on and enjoy the winning article on the topic Biosafety & R&D – Important But Ignored Aspect. Congratulations once again.
Biosafety & R&D – Important But Ignored Aspect
1. A dissertation trainee prepares 100ml, 0.1N acetic acid solution from a 1N acetic acid stock solution by pipetting 10ml from the latter solution and adding it to 90ml of water.
2. At 13.00 hrs, a researcher in microbiology quickly places all the culture slant tubes in cold storage and then rushes out of the laboratory to grab a quick bite in the nearby refreshments area.
3. After completing a practical workshop on haematology, all participants diligently discard their used lancets into the waste bin.
4. After succeeding at separating a desired analyte using a liquid chromatographic approach, a chemist carefully discards the unused solvents and acidic buffers of the mobile phase into the drain.
All the above incidents appear as a part of routine bench work in some laboratories. Nothing seems unusual except that in the first instance, mouth pipetting was carried out
, in the second case, the researcher was too hungry to remove the lab coat before rushing to eat, non-sterile, used and potentially biohazardous lancets were dumped together with other general waste in the third case while in the fourth scenario, poorly degrading solvents and corrosive acidic buffers were released into the sewage line mainly meant for water and other organic biodegradable wastes.So what is biosafety anyway and why is it not on every researcher’s mind?
As per the United Nations Environment Programme, biosafety is the prevention of large scale loss of biological integrity, focusing both on ecology and human health [1]. In other words, biosafety helps to avert harmful incidents. It involves continuous risk management assessment and stringent enforcement protocols for biosafety. A few of the reasons for turning a blind eye to biosafety could be:
- A deep-rooted and age-old approach of “research first” or “product first” that prioritizes results or data over safety.
A life science graduate is made aware of research methodology, design and planning of experiments, troubleshooting and result analysis. But seldom do we find such graduates receiving training to look up the relevant material safety data sheets (MSDS) pertaining to the chemicals and reagents that they regularly handle. In fact, few R and D laboratories would have a well-maintained file of relevant MSDS for easy and quick access by researchers.
2. Lack of a “system” approach to biosafety
The existing safety protocols address isolated safety concerns rather than being a holistic and harmonized approach to overall safety.
3. Lack of government regulations
In absence of proper government guidelines pertaining to safety at academic R and D institutions, biosafety gets compromised. This is in contrast to large scale production facilities where safety guidelines are clearly and strictly laid down. e.g., The Factory Act, 1948 [2].
4. Lack of appropriate and complete safety training
All new joiners and trainees need to be imparted with mandatory safety training before joining the laboratory along with reading and understanding of all safety standard operating procedures (SOPs).
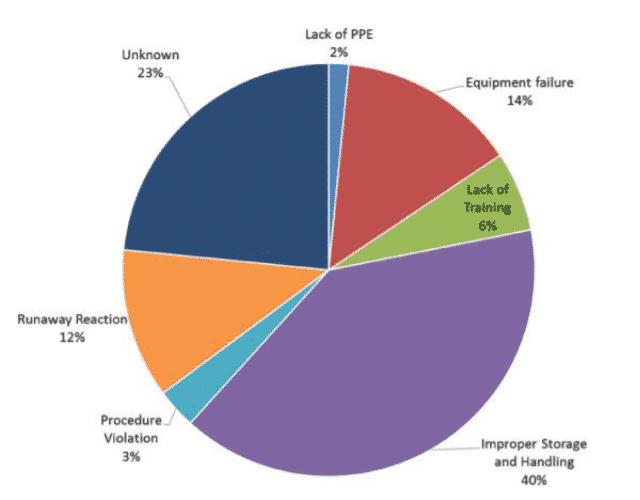
A. Change in approach:
A shift is needed from research first or product first to a safety-first mindset. Safety needs to be ingrained as a value similar to data integrity. For instance, while planning an experiment, a researcher could imagine what all things could possibly go wrong and impact safety. Adopting a bottom-up approach and introducing Environmental Health and Safety Studies (EHSS) at the undergraduate level could help in inculcating a habit of safety.
B. Setting up a hierarchy of controls and checks:
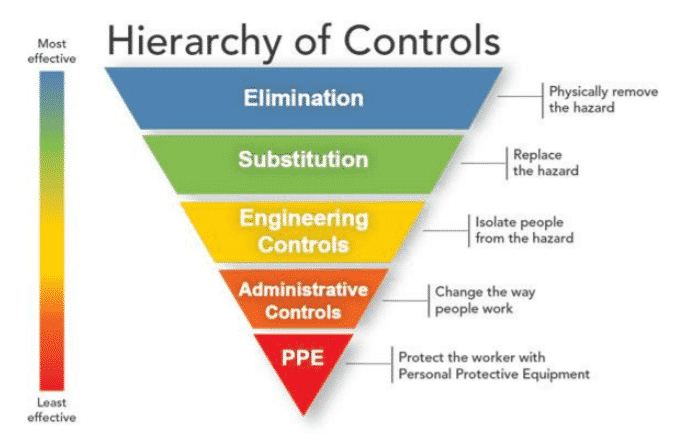
options like substitution, engineering controls, administrative controls and personal protective equipment (PPE) can also be explored:
i. Elimination – This involves the physical removal of the hazard itself, e.g., decommissioning of leaking or faulty equipment and instruments, mandatory switching off main regulator valves of all gas lines at the end of the day.
ii. Substitution – This includes replacement of the hazard, e.g., use of silver salt and hydrogen peroxide for fogging in microbiological areas instead of fumigation with a combination of potassium permanganate and potentially carcinogenic formaldehyde.
iii. Engineering controls – This involves isolation of people from the hazard, e.g., use of fume hoods, isolators and biosafety level 3 and 4 cabinets while working with obnoxious chemicals, cytotoxic agents or pathogens respectively, installation of fire alarm systems along with fire extinguishers, construction of the facility for containment of radiological wastes.
iv. Administrative controls – Here the idea is to change the way in which people work, e.g ., the use of mechanized pipetting pumps for withdrawing liquid reagents or solutions, restraining gas cylinders with chains and transporting them using carts.
v. Personal protective equipment (PPE) – The worker is protected from the hazard using protective equipment, e.g., use of hand gloves, face masks, lab coats/ aprons/ gowns, lab footwear, head cover, protective glasses.
Besides, safety can be in terms of process, occupation or environment.
C. System approach to safety and training:
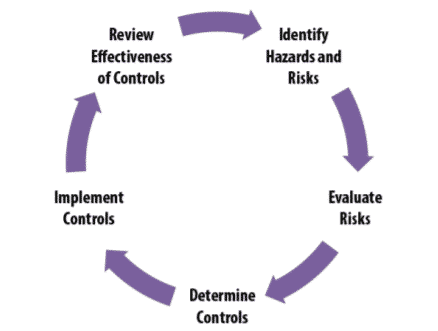
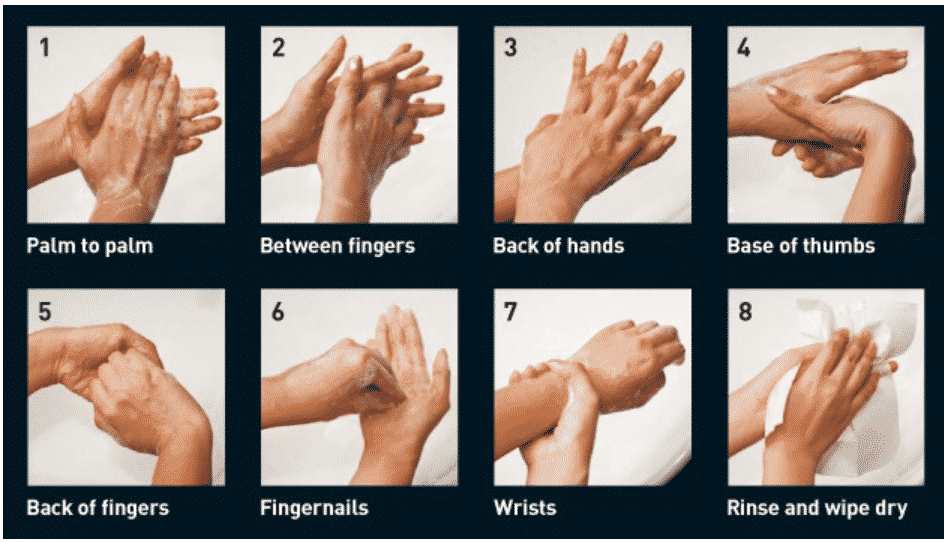
As part of laboratory hygiene, no member should ever work all alone in the laboratory. The 8-step hand washing protocol should be encouraged. A well-stocked first-aid kit should be readily available in the laboratory at any given time. A telephone can be placed in a designated area along with emergency contact numbers displayed near it.
inventory. Ideally, no chemical should be stored above eye level and they need to be arranged as per compatibility. Only working volumes of solvents can be stored near the workbench. Separate mild steel cabinets can be allocated for corrosive agents, acids and oxidizing agents.
All instruments and equipment should be accompanied by operation and safety instructions with respect to voltage, electrical earthing, pressure, temperature, vibrations, radiations.
As and when required, identification of need for training followed by imparting such training
and then its evaluation needs to be undertaken. Hands-on training on first-aid and regular mock drills on safety would be helpful.
D. Waste disposal:
- Laboratory wastes can be segregated as chemical and biological/ biohazardous waste and each of them can be collected separately. Ideally, biowaste collection should be entrusted to a specific third party vendor who is well qualified and certified for handling such waste. In fact, no waste should be dumped on any unauthorized collectors.
- All microbial wastes should be appropriately decontaminated at 15 psi, 121 degrees Celcius for 30 minutes before disposal. If necessary, incinerators can be used. Special arrangements (pits) have to be made for the containment of radiological wastes if they are generated at any facility. Glass and sharp wastes should be discarded separately.
- Solutions with extreme pH and corrosive reagents should not be released directly into the general sewage system.
E. Accident reporting:
- Any kind of safety glitches or accidents should be promptly and honestly reported to the
concerned safety team. This could serve as raw data during the revision of safety plans. - External safety audits, as well as internal self-audits, need to be organized at regular intervals.

Overall, a passion for safety with the mandate that “safety is everyone’s duty” needs to be encouraged. Most importantly, safety is not the responsibility of the trainees and safety officers alone and so all members of any laboratory need to abide by the safety rules and thus lead by action and example rather than just spelling out mere words on safety. Besides, there is no “just this one time” in the case of biosafety since accidents can happen any time and so all safety measures need to be diligently followed at all times without any exceptions.
References:
- Biosafety and the environment: An introduction to the Cartagena Protocol on Biosafety (PDF). GE.03-01836/E. United Nations Environment Programme. p. 8.
- https://labour.gov.in/industrial-safety-health
- Gopalaswamy, N., Han, Z. (2020) Analysis of laboratory incident database. Journal of Loss Prevention in the Process Industries. Volume 64, March 2020. https://www.sciencedirect.com/science/article/abs/pii/S0950423019302153
- https://www.cdc.gov/niosh/topics/hierarchy/default.html
- https://www.cdc.gov/safelabs/resources-tools/bio-risk-assessment.html
- https://www.healthhub.sg/live-healthy/471/keepyourhandsclean
- https://www.carolina.com/teacher-resources/Interactive/infographic-lab-safety-rules/tr35303.tr
Sources:
- “Building a Safe and Resource Efficient Laboratory”, EHS Webinar, NBM Webinar Series 2020, 12 November 2020.
- “Developing Skills and career as EHS Professional in India”, EHS Webinar, NBM Webinar Series 2020, 26 November 2020.
 Sulagna Roy holds a post-graduate degree in Microbiology from The Maharaja Sayajirao University, Vadodara. Her functional role as an R&D microbiologist has so far allowed her to explore various types of microbes such as Actinomycetes, fungi and bacteria. Her chief interest lies in upstream microbial fermentation and industrial microbiology. She has an understanding of microbial culture preservation and management, mass cultivation of microbes using submerged or solid-state fermentation, process parameter optimization for improving secondary metabolite production in microbes.
Sulagna Roy holds a post-graduate degree in Microbiology from The Maharaja Sayajirao University, Vadodara. Her functional role as an R&D microbiologist has so far allowed her to explore various types of microbes such as Actinomycetes, fungi and bacteria. Her chief interest lies in upstream microbial fermentation and industrial microbiology. She has an understanding of microbial culture preservation and management, mass cultivation of microbes using submerged or solid-state fermentation, process parameter optimization for improving secondary metabolite production in microbes.






























wow, thank you