Coronavirus Vaccine Mechanism
Scientists around the world are working around the clock to manufacture a risk-free and effective COVID-19 vaccine. Currently, a handful of vaccines have been approved and are being provided to the public worldwide; a lot more vaccines are under development.
Vaccine development involves various steps to ensure the final product is both safe and efficient. Initially, the fundamental research is carried out, once the vaccine candidate is manufactured, preclinical animal studies are conducted in animal models. Then the vaccine enters small phase 1 studies, focusing on safety, and then larger phase 2 studies, with a focus on efficiency.
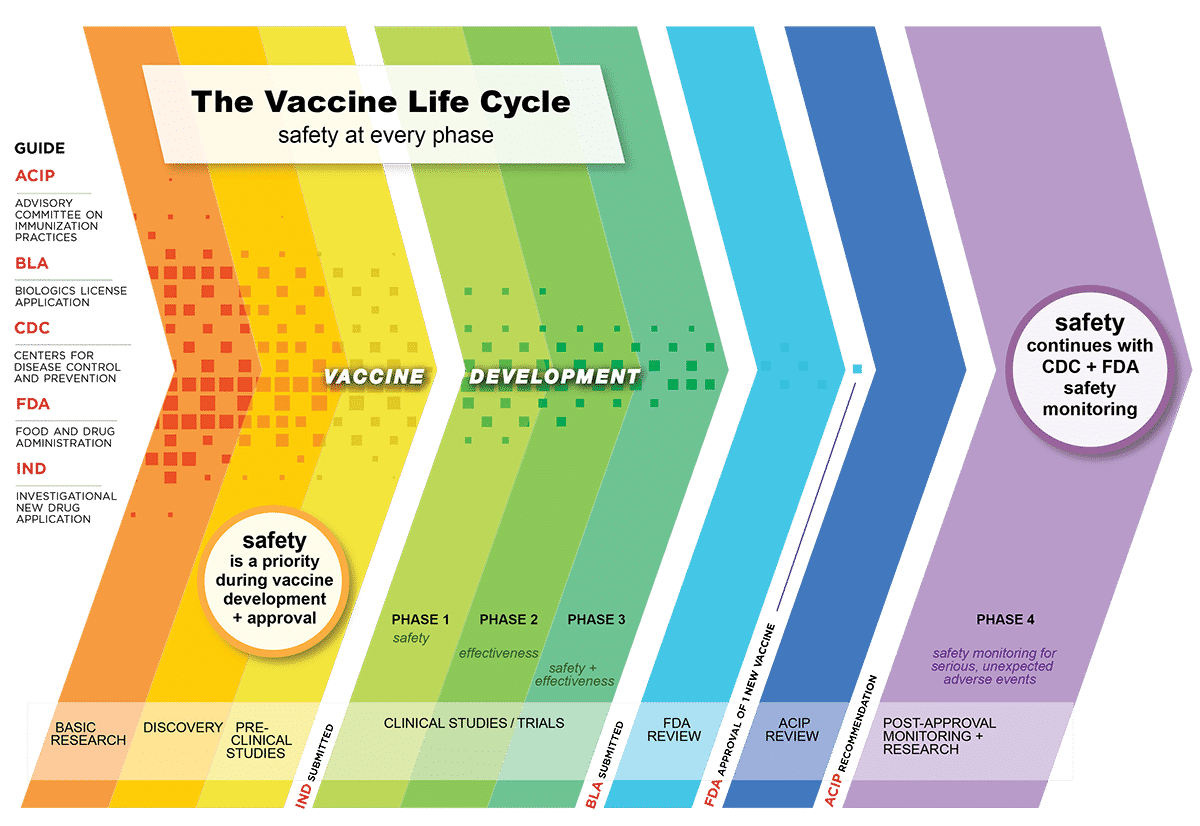
The urgency to develop a COVID‑19 vaccine has forced to compress schedules that reduced the regular vaccine development timeline. Many steps have been overlapped with each other to curtail the timeline. Normally, developing a vaccine takes around 15 years or more. However, COVID vaccine development has been cut shortened largely to fight this pandemic.
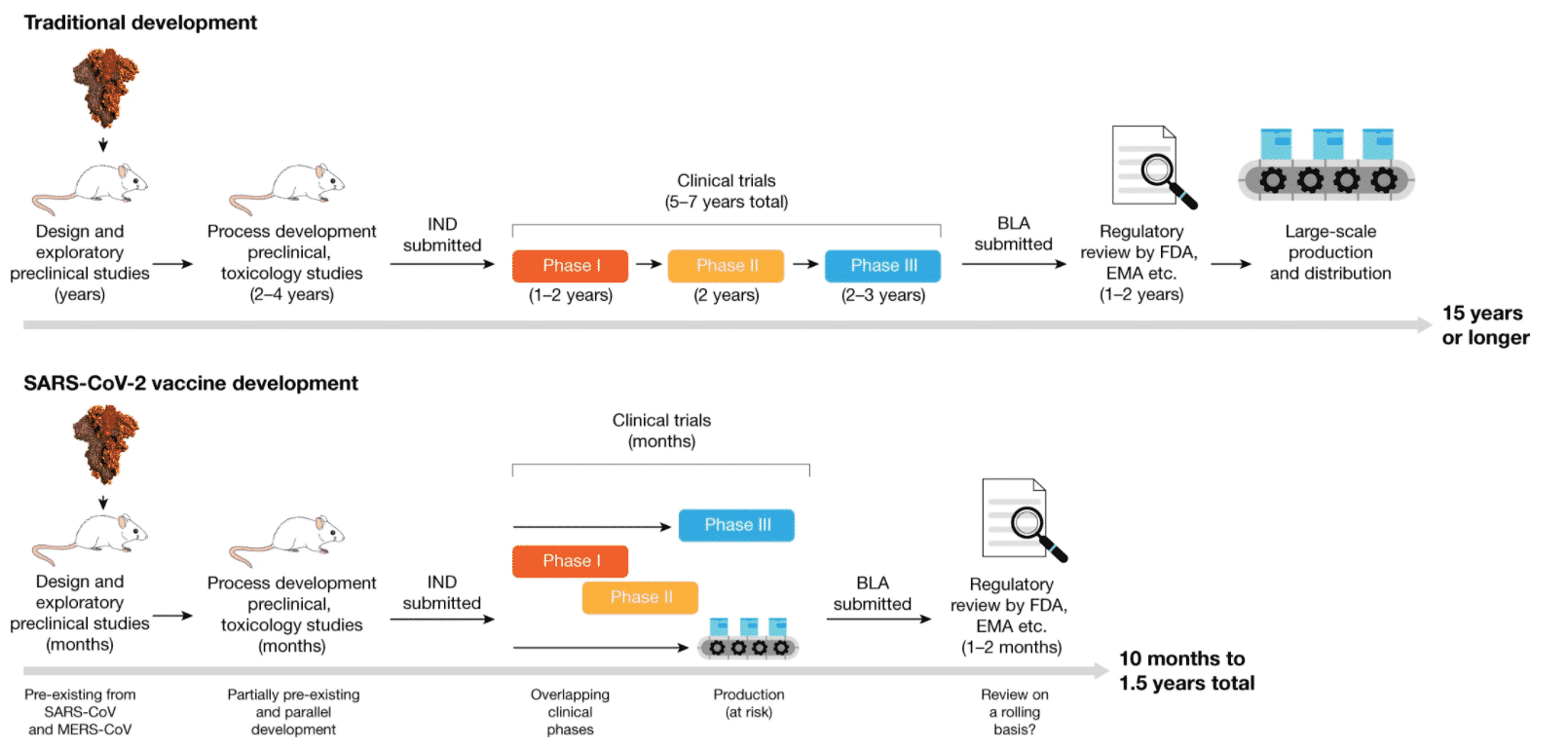
Table of Contents
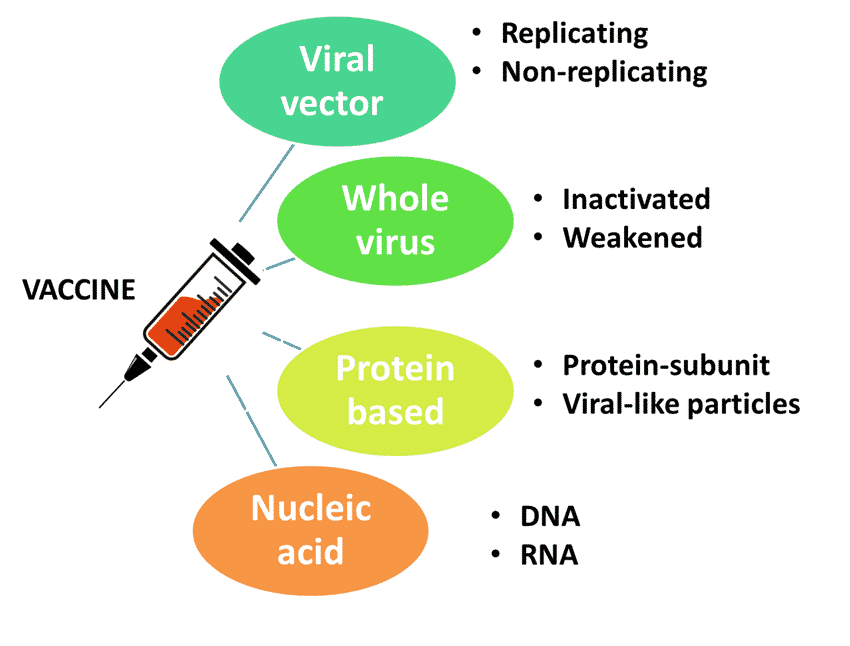
Different types of COVID-19 vaccine
Several COVID-19 vaccines are under development; favorably, some vaccines have shown positive outcomes in phase
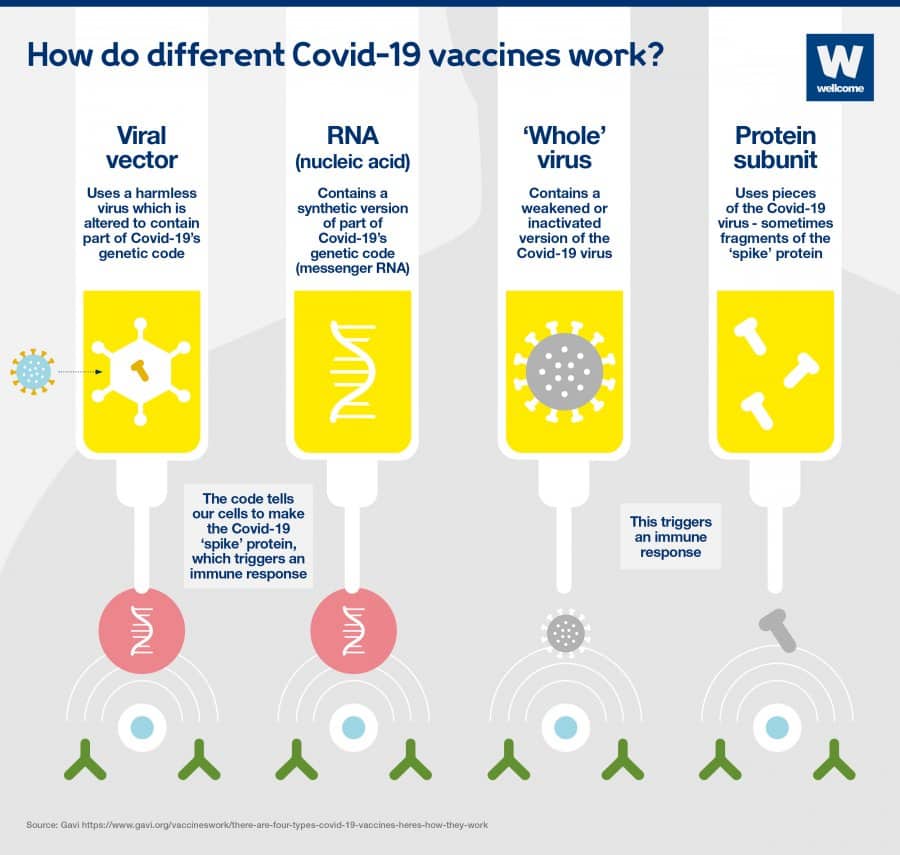
III clinical trials. Now, let’s see how different vaccines work and Coronavirus Vaccine Mechanism?The principle behind all vaccines is – stimulating the immune system to produce an immune response that is usually similar to that presented by the natural infection in a risk-free means. Vaccination teaches our body to recognize and eliminate the pathogen.
Vaccines can be manufactured by different methods. Different types of vaccines are discussed below:
Coronavirus Vaccine Mechanism
-
Viral vector vaccines
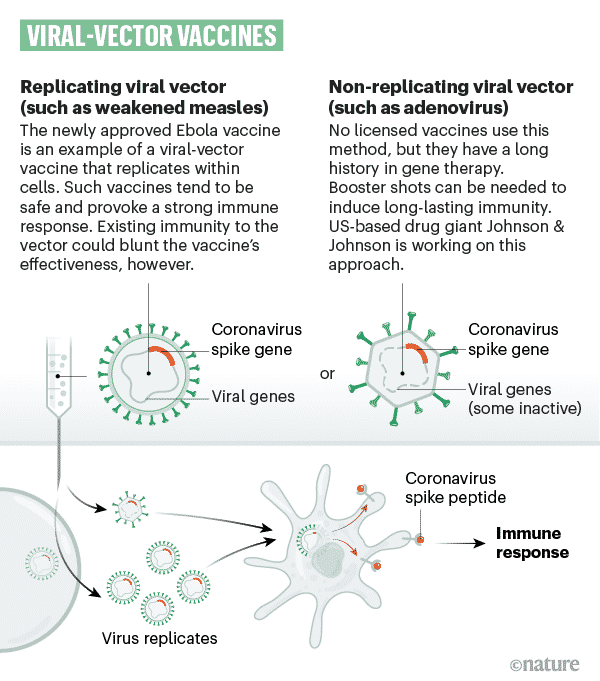
A safe virus is modified by introducing part of the disease-causing virus’ gene sequence, like the gene sequence for COVID-19’s ‘spike’ protein. Viral vector vaccines use part of a different virus, the one that has been genetically altered not to be infectious.
The safe virus transports the gene sequence into our cells, which then start to synthesis the protein, triggering an immune response, preparing our immune system to attack the real virus in the future. The gene sequence only contains info to make a single COVID-19 spike protein, one to trigger your immune system; however, it won’t cause COVID-19.
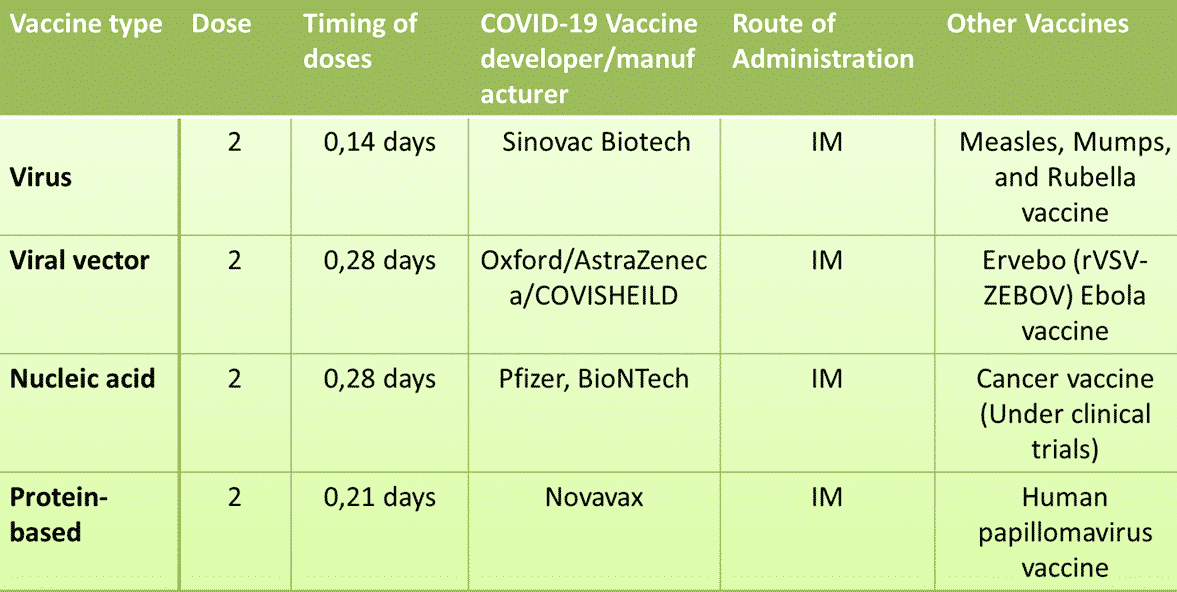
Oxford-AstraZeneca or Covisheild vaccine is manufacture based on the viral vector vaccine technology. Another important fact about this vaccine is that it is the first viral vector vaccine to be approved for COVID-19. Few examples of non-replicating viral vector vaccines for COVID-19 under development are CanSino Biologics, Gamaleya Research Institute, Janssen, ImmunityBio, Inc. & NantKwest Inc., ReiThera / LEUKOCARE / Univercells, CanSino Biological Inc / Institute of Biotechnology, Academy of Military Medical Sciences, PLA of China, Vaxart, Ludwig-Maximilians – University of Munich. All of these vaccines use adenoviruses as the vector.
From a scientific perspective, these vaccines can be classified into viral vectors that can make copies of themselves in the body (replicating viral vectors) and those that can not (non-replicating viral vectors). However, the principle behind both is the same. The examples of replicating vector COVID-19 vaccines are developed Beijing Wantai Biological Pharmacy/ Xiamen University – An Intranasal flu-based-RBD vaccine, Merck Sharp & Dohme/IAVI, and Institute Pasteur / Themis / Univ. of Pittsburg CVR/Merck Sharp & Dohme.
-
Nucleic acid vaccines
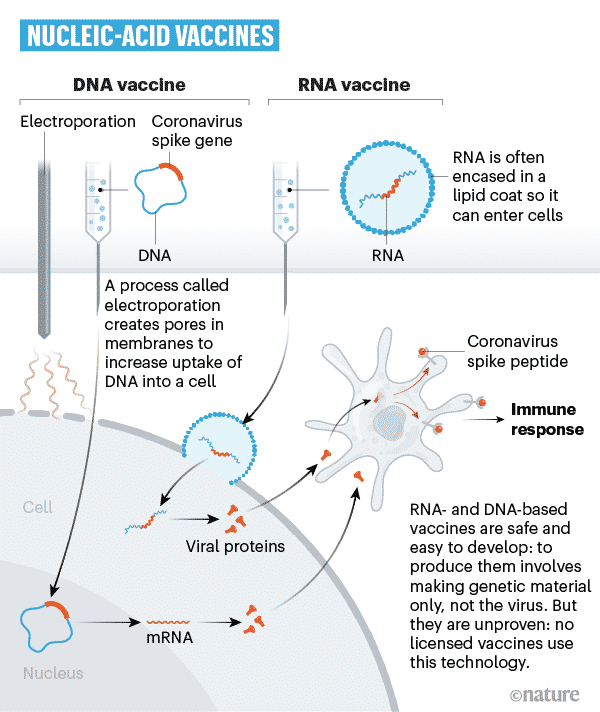
Recent vaccine technologies are developed around nucleic acids: DNA and mRNA.
Such vaccines use a small part of mRNA or DNA synthesized in a laboratory to eventually activate an immune reaction. This small part of the gene has the code for the specific viral protein required, like the COVID-19 spike protein.
The gene product enters the body’s very own cells using certain carrier particles added to the vaccine. Then the body’s cells use this genetic data to synthesis the real viral proteins. The body’s cells will be used to create a type of protein usually made by the virus. However, this won’t cause infection.
Some of your cells will produce a little COVID-19 spike protein along with various other proteins your body necessitates day-to-day. This will certainly trigger your body’s immune system so that it can fight off the virus. To manufacture an RNA vaccine, researchers create a synthetic variant of the virus’ mRNA.
When this is injected into our body, cells review it as an instruction to start building the pertinent viral protein. This triggers our immune system to react, and in doing so, it learns how to protect from future COVID-19 encounters.
RNA COVID-19 vaccines are Pfizer-BioNTech and Moderna’s vaccines; Curevac; Arcturus/Duke-NUS; Imperial College London; and People’s Liberation Army Academy of Military Sciences/Walvax Biotech. Both have recorded extraordinary levels of vaccine effectiveness of about 95%.
They are the 1st RNA vaccines ever to be authorized for usage versus any type of disease. However, scientists have actually been using the innovation for a while and have been used in human clinical trials for diseases like cancer.
Vaccine candidates that use the DNA vaccine approach are the vaccines developed by Inovio Pharmaceuticals/ International Vaccine Institute; Osaka University/ AnGes/ Takara Bio; Cadila Healthcare Limited; and Genexine Consortium.
-
‘Whole’ virus vaccines
Whole virus vaccines can be developed by two different methods. One of them is the Live virus vaccine/weakened, which employs a virus that is alive and active to stimulate an immune reaction. Nonetheless, the virus has been altered and severely weakened to make sure that it doesn’t cause any severe diseases.
The vaccine used against measles, mumps, and rubella vaccine (MMR) is an example of live, weakened virus vaccines.
As these viruses are still alive, these types of vaccines require more comprehensive safety screening. They might be most likely to trigger considerable adverse events compared to those made by various other approaches.
Such injections might not be safe for individuals who have weakened immune systems, either from taking specific medicines or due to some medical conditions.
This method’s benefit is that they tend to trigger a really solid immune reaction that lasts longer. It is less complicated to produce a one-shot vaccine utilizing a live virus vaccine than with other vaccines.
Another type of the whole virus vaccine is Inactivated vaccines, one of the first kinds of general vaccines to be developed. They are developed by killing the pathogen to inactivate it. Then, it is injected into the body to trigger the immune system.
As the virus is dead, it can not certainly infect you, even if you have any hidden immune system problems. This type of vaccine triggers long-term immunological memory that helps protect you if you’re ever before exposed in the future.
Generally, inactivated vaccines usually need multiple doses. But, they are safe and more stable to work with than with live viruses vaccines.
Some of the most advanced COVID-19 vaccines under development include Sinovac; Bharat Biotech; Sinopharm; Institute of Medical Biology, Chinese.
Academy of Medical Sciences; Research Institute for Biological Safety
Problems, Rep of Kazakhstan; Beijing Minhai Biotechnology Co., Ltd.
The vaccines for whooping cough, rabies, and hepatitis A are a few examples of existing inactivated vaccines.
-
Protein subunit vaccines
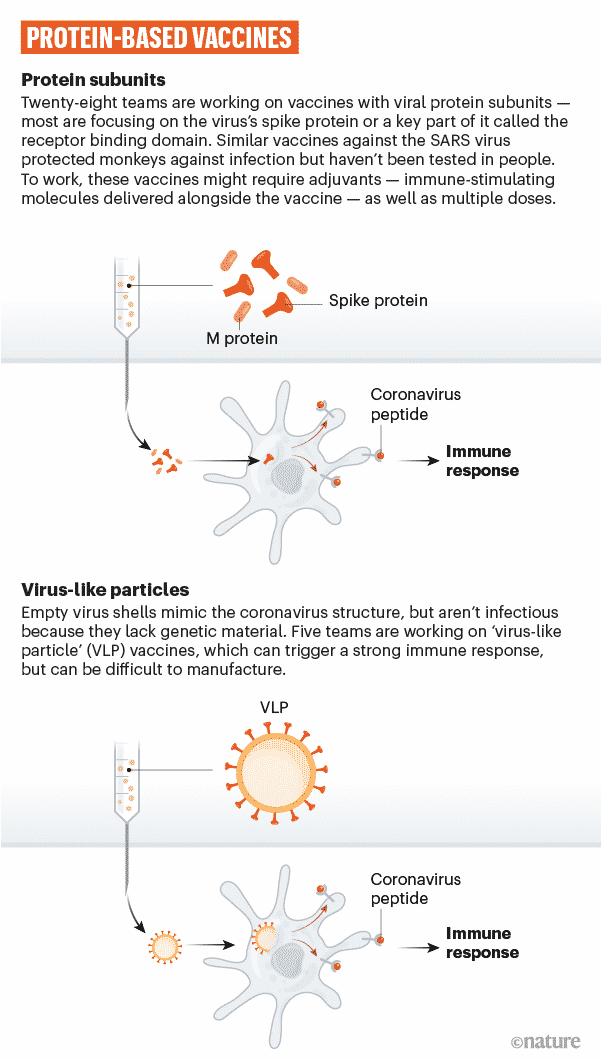
A short piece of the viral gene sequence is inserted into another cell like a bacterial, yeast, mammalian, or insect cell – the genetic code contains details for this cell to begin building the viral protein.
Such cells serve as factories, building huge amounts of the proteins, which are later extracted, purified, and utilized as the vaccine’s active component.
When such vaccines are injected into our body, the body’s immune system learns to recognize the viral protein so that it can produce an immune response that protects against future infection.
COVID-19 vaccine candidate which uses protein subunit approach are Anhui Zhifei Longcom Biopharmaceutical / Institute of Microbiology, Chinese Academy of Sciences; Kentucky Bioprocessing, Inc; Sanofi Pasteur / GSK; Biological E Ltd; Clover Biopharmaceuticals Inc. / GSK /Dynavax; Vaxine Pty Ltd / Medytox; University of Queensland / CSL / Seqirus; Medigen Vaccine Biologics
Corporation/NIAID/Dynavax; Instituto Finlay de Vacunas, Cuba; FBRI SRC VB VECTOR, Rospotrebnadzor, Koltsovo; and Novavax and the Chinese Academy of Sciences.
Seldom a certain protein, which is considered to be a good trigger for the immune system, is extracted and purified from live viruses. In some cases, researchers manufacture the protein themselves (to one that is almost similar to a viral protein).
This laboratory designed and manufactured protein is called a “recombinant” protein. Hepatitis B vaccine is an example protein subunit vaccine, which is already being used.
Another type of protein-based vaccine is vaccines developed by virus-like particles. These include several structural proteins from the virus, not the virus’s genetic material. An example of this kind of vaccine is the human papillomavirus vaccine.
SpyBiotech and Serum Institute of India’s COVID-19 vaccine is an example of a viral-like particle vaccine.
Keywords: Coronavirus Vaccine Mechanism; Mechanism by which coronavirus vaccine work;