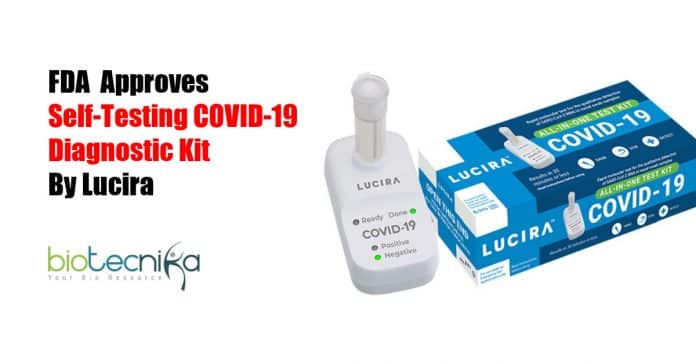
Self-Testing COVID-19 diagnostic Kit Authorized by FDA
Adding an extra tool to fight this COVID-19 pandemic as national testing capacities come under more pressure. The United States approved the first COVID-19 diagnostic kit for self-testing at the comfort of your home. As per the govt officials, the FDA provided an emergency use authorization (EUA) to rapid-result All-In-One Test Kit by Lucira Health Inc.
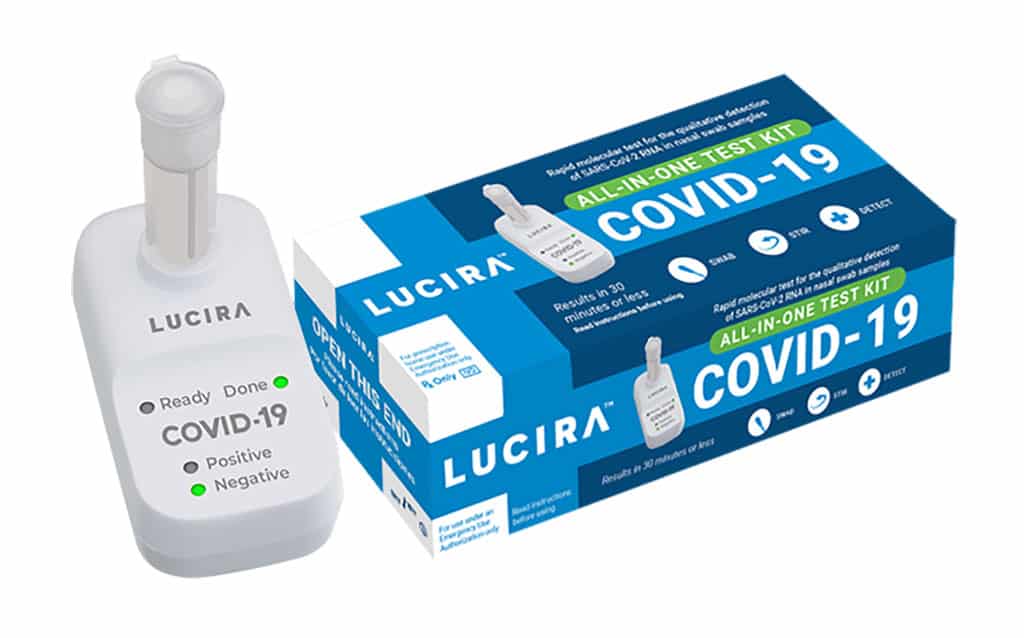
What is the Lucira test?
Presently, the Lucira test is commissioned for prescription use only, and the FDA stated that the health-care providers should report all outcomes to the government. The Lucira test functions by rolling the self-collected sample swab in a vial that is placed in the testing unit. The testing unit’s light-up display will show the results of the test. Along with the use in houses, the product is accredited for usage in medical professionals’ workplaces, medical facilities, and emergency clinics.
What is the difference between the Lucira test and other COVID-19 tests?
Some COVID-19 tests allow individuals to give samples from the house, but this self-testing COVID-19 diagnostic kit is the very first test that can be completely self-administered and gives results at your
house within half an hour or less. The authorization comes with a time when the nation – which has the highest number of total cases worldwide (above 11 million) – is combating an intense resurgence of cases.Additionally, this test can also help take off some burden from the testing laboratories that are being flooded by demand. However, full self-testing is not commonly adopted worldwide as it runs the risk of human mistakes, and incorrect outcomes to occur, and cases may go unreported to the authorities.
Stephen Hahn, the FDA commissioner, stated that this novel screening option is a great diagnostic advancement to address the pandemic and minimize the public worry of disease transmission.
Keywords: Self-Testing COVID-19 diagnostic Kit Authorized by FDA