First 3D map of heart’s neurons
Our body’s control center – the brain maintains the regular performance of our hearts through an elaborate nerves network. Heart problems, including heart attacks, sudden heart death as well as issues in the supply of blood is caused when this communication is interrupted. The heart has its own ‘little brain’ as an added layer of safety to monitor as well as correct any kind of local disturbances in communication – known as the intracardiac nervous system (ICN). The ICN is crucial in sustaining heart health as well as during heart attack it can protect heart muscle. How specifically the ICN performs these functions is yet not clear because the organization of the neurons that compose the ICN is poorly understood. We do not know where they are located in the heart, what their molecular properties are, and how they are attached to each other.
A 3D map of heart’s neurons
Scientists from Thomas Jefferson University and their partners have been able to address these questions
in extraordinary detail. The research is released in the journal of iScience.James Schwaber, Co-senior author, Director, the Daniel Baugh Institute for Functional Genomics and Computational Biology (DBI), said “The ICN represents for a big void in our understanding that falls in between cardiology and neurology”. “The objective was to bridge that void by supplying a physiological framework of the ICN”.
Raj Vadigepalli, co-senior, Ph.D., Professor of Pathology, Cell Biology, and Anatomy said, “The only other organ for which such a detailed high-resolution 3D map exists is the brain”. “What we have created is the initial comprehensive roadmap of the heart’s nervous system that can be referenced by various other researchers for a range of concerns regarding the ICN’s function, physiology, and connectivity of different nerve cells”.
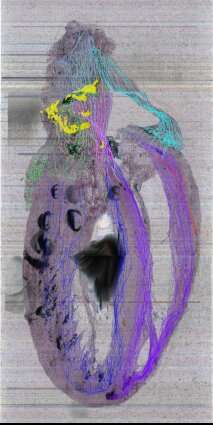
Producing a dual-approach pipeline, technologies, and expertise from various research study teams (from Jefferson and the University of Central Florida) and industry companions (Strateos and MBF Bioscience), was used in the research. An approach allowed the scientists to construct an exact 3D version of the entire rodent heart, the approach is a novel imaging strategy called Knife-Edge Scanning Microscopy (KSEM). This technology is used for the first time for the cardiac study. The second method used a method called laser capture microdissection to sample single nerve cells for gene expression evaluation, in addition, to map their individual positions within the heart’s 3D framework.
Sirisha Achanta, Lab Manager at the DBI and co-author said, “We were trouble-shooting the protocol as we went along as this hasn’t been done prior.” “We needed to figure out methods to preserve uniformity across each heart that we imaged, as the heart is not symmetrical unlike the brain”.
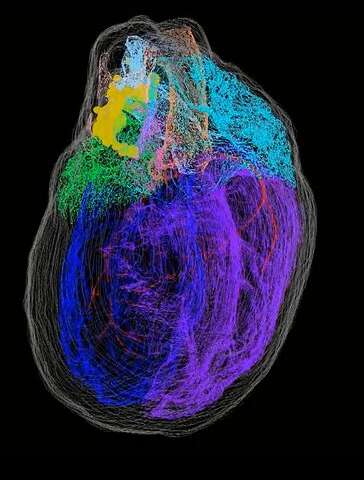
Unknown intricacies of the ICN were revealed by the 3D map of the heart’s neurons. The scientists discovered that the nerve cells that make up the ICN are located in a coherent band of clusters on the base (top) of the heart, where the heart’s blood vessels and arteries enter and leave. However, it also extends on the heart’s back – down the length of the left atrium. They’re placed close to particular vital heart frameworks like the sinoatrial node.
Jonathan Gorky, co-author, a recently graduated MD/Ph.D. student and a resident at the Massachusetts General Hospital, said “We know that in developing the heart pace or rate, the sinoatrial atrial node is important”. “The neuron clusters around it was something we had always presumed however had never known. To see the physical evidence of the ICN’s feature as well as the accurate distribution of the neurons in connection to the heart’s anatomical frameworks was actually intriguing”.
The studies of gene expression of individual nerve cells also pointed to previously unidentified variety of molecular phenotypes or identities. Dr. Vadigepalli explains “We discovered that there are several types of neuromodulators and receptors”. “This implies that we don’t simply have nerve cells in the heart that turns on and off the activity, yet additionally those that can fine-tune the activity of the ICN”.
The scientists also discovered sex-specific differences in the way neurons were arranged, both spatially and by their gene expression, when comparing male and female rat hearts. More evaluations are pursued on the basis of these findings by the co-authors Shaina Robbins, senior research assistant, and Alison Moss, Ph.D. Candidate. Alison Moss said, “Several of the differences in men and women ‘s heart disease can be described by this study”. “To explore those questions further, we’re currently trying to develop a 3D design of the innate nervous system of the pig heart, which is a lot more anatomically similar to the human heart”.
The project is part of an NIH study program called SPARC – Stimulating Peripheral Activity to Relieve Conditions. This program intends to promote the development of therapeutic devices that regulate electric activity in nerves to enhance the function of the organ. Dr. Schwaber said, “About 30 years earlier, there were researches showing peripheral nerves like the vagus nerve were vital for stimulating organs as the heart could even rectify disease condition and the health of organs”. “Yet those studies were unable to inform us what amount, frequency, as well as the place of stimulation, is dangerous or advantageous”.
Dr. Vadigepalli said, “Since we understand where nerve cells are located in relation to heart frameworks, we can ask concerns such as – does stimulating in one area, or perhaps selectively particular nerve cells make a distinction?”.
Various other groups in the SPARC is using the pipeline produced by scientists. Achanta said, “The procedure is extremely reproducible and is readily available for other organ systems to map not just nerve cells, but other micro-structures as well as it uses day-to-day lab materials and techniques”.
Dr. Schwaber said, “Ultimately the aim is to develop a 3D map for the human heart for health and disease”. “We have developed the groundwork for an unlimited possibility of researches in the future“.
Author: Sruthi S