World’s First Oral TRK Inhibitor By Loxo – Bayer Gets FDA Nod
Loxo Oncology Inc’s first commercial medicine turns out to be the first ever Oral TRK Inhibitor to get a green signal from FDA for the treatment of NTRK (neurotrophic receptor tyrosine kinase) gene fused solid tumors in both infants & adults for which no treatment exists till date. In partnership with Bayer AG “Vitrakvi” (larotrectinib) will be sold in the market priced at $32,800 per month.
As per the Press release, Vitrakvi is the first ever treatment to get a tumor-agnostic indication at the time of initial FDA approval. In clinical trials of patients with TRK fusion cancer, Vitrakvi demonstrated an ORR of 75 percent (N=55) (95% CI, 61%, 85%), including a 22 percent complete response (CR) rate. The clinical trial was conducted on a total of 22 patients, wherein the oral inhibitor showed a drastic reduction in tumor among 81 percent of the patients who earlier showed symptoms of 24 different types of cancer, namely lung cancer, pancreatic cancer, breast cancer, and soft tissue sarcomas. Adding to this incredible result minimal side effects were noted.

Vitrakvi was reviewed by FDA under priority review category, which is reserved for drugs and treatments that could provide significant
improvements in treating serious and lethal disease & medical conditions.Josh Bilenker, M.D., chief executive officer of Loxo Oncology in a statement said: “We are grateful to the investigators, research teams and patients who contributed to and participated in the larotrectinib clinical trials that supported this approval,”.
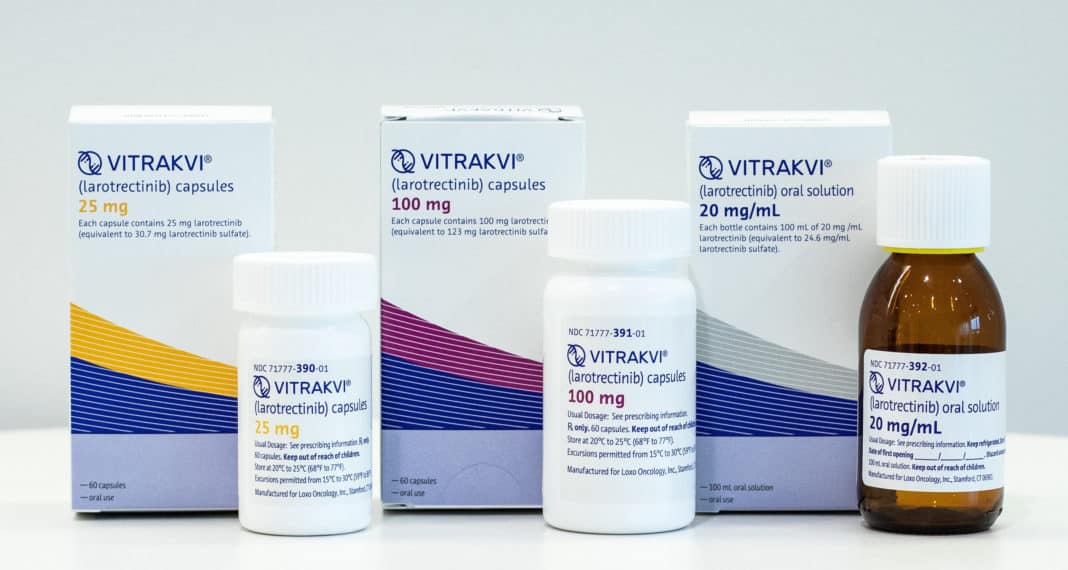
Vitrakvi will be made available in the form of oral capsules as well as a liquid formulation for both adults and children. Visit www.vitrakvi.com for more information.
As per Loxo’s future projects are concerned, it is developing a drug, LOXO-292 targeting another set of rare gene fusion mutation known as RET, seen in lung cancer, thyroid cancer, and others as well. Also teamed up with Bayer they have a follow-up TRK drug in development known as LOXO-195.
In past similar type of drugs have been developed by Merck and Roche, leading to an increase in demand for advanced genetic testing of tumors in order to identify the patients likely to benefit from the treatments.