Researchers Map Telomerase in More Detail than Ever Before
Why do we get old? It is, on the face of it, a puzzle. Cells can effect repairs, and divide to make new copies of themselves – they’ve been doing it for four billion years, give or take. So why do the cells in our bodies wear out? Why, after a few short decades, do they stop working?
The search for the key to ageing – and a means of slowing or reversing it – is far older than anything we’d call science. In ancient China around 200 bc, a Qin emperor sent an alchemist Xu Fu to the eastern seas to find the elixir of life; medieval alchemists sought the “philosopher’s stone” which, as well as transmuting base metals into gold, was said to prolong the life of anyone who ate a piece of it. A more scientific version of this quest is still going on.
For years now, it’s been said that telomeres – the tips of your chromosomes – are the key to cancer and aging. The shorter they are, the worse off you are – so the story goes. But what do we really know about them?
Now, in the most detailed map
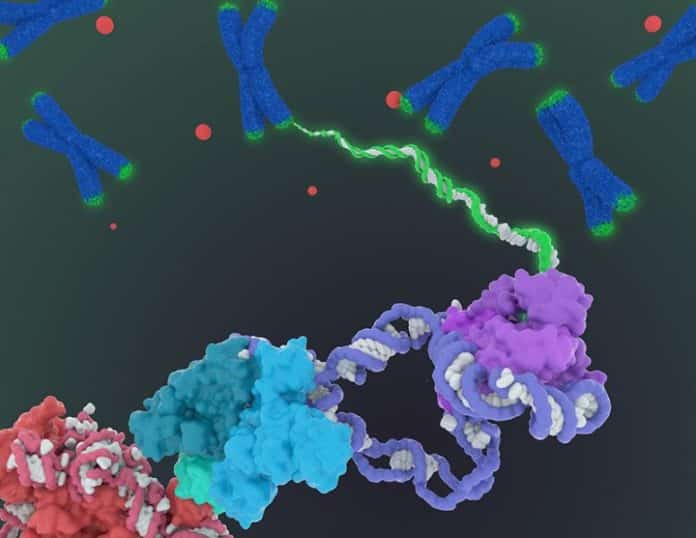
of telomeres yet, researchers at UC Berkeley have presented the cryo-electron microscopy structure of the substrate-bound human telomerase holoenzyme at subnanometre resolution, showing two flexibly RNA-tethered lobes: the catalytic core with telomerase reverse transcriptase (TERT) and conserved motifs of telomerase RNA (hTR), and an H/ACA ribonucleoprotein (RNP).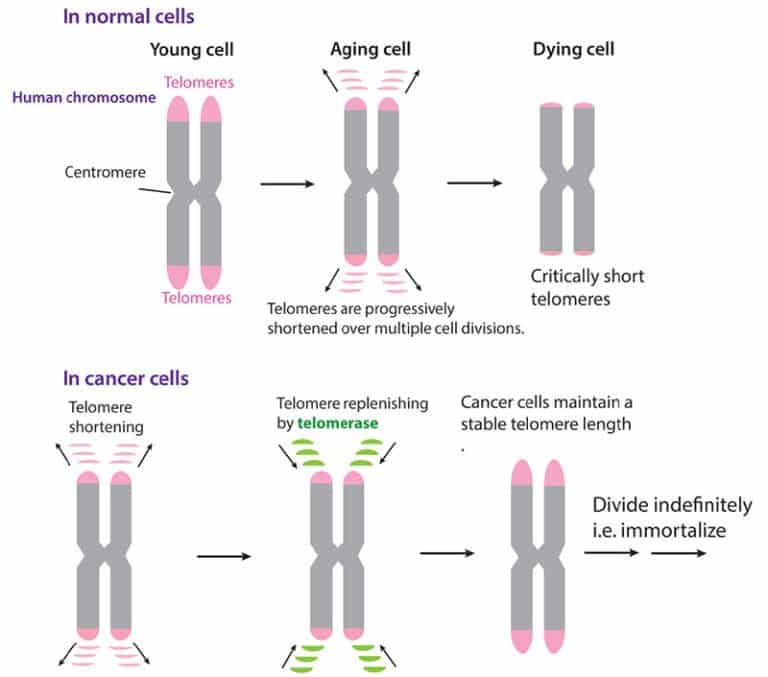
Telomerase was discovered in 1997, but it’s so complicated that researchers have been unable to determine what it looked like until now. The researchers used a new imaging technique called cryo-electron microscopy (cryo-EM), which involves cryogenically freezing a sample before looking at it with an electron microscope. This technique can illuminate structures too sensitive for normal electron microscopes.
Telomerase has an exceptionally complex structure, with an RNA backbone decorated by six types of protein that move around as they add DNA to the ends of chromosomes.
With the complex structure comes complex questions, such as whether the enzyme operates singly or as conjoined twins, and how and just how many proteins decorate the RNA backbone.
Without answers to these questions, it has proven difficult to design a drug to target the molecular machine and either destroy telomerase activity, or indeed restart telomerase, such as to boost cell division after a bone marrow transplant.
The Berkeley lab has been trying to determine the structure of telomerase for 20 years and had made some advances before now, including discovering and characterising many of the proteins in the enzyme, as well as the broken-up hairpin structure of the RNA backbone of telomerase.
The newly revealed structure still lacks fine detail, but combined with knowledge of the gene sequence of human telomerase, it provides enough information to start thinking about potential targets for drugs, said first author Thi Hoang Duong “Kelly” Nguyen, a Miller Institute postdoctoral fellow at UC Berkeley.
“The best previous images of human telomerase had a resolution of only 30 Ångstroms; we were able to get about 7 to 8 Ångstroms resolution using cryoelectron microscopy,” Kelly said. “When I got to the point where I could see all the subunits – we had 11 protein subunits in total – it was a moment of, ‘Wow, wow, this is how they all fit together.’”
Nguyen was able to isolate the active enzyme and purify it much better than anyone had before, and employed a new, state-of-the-art cryoelectron microscope to determine the structure of the active telomerase unambiguously. Cryo-EM is a technique for determining molecular structures of compounds that cannot be crystallized and imaged with X-rays, and its developers won the 2017 Nobel Prize in Chemistry.
The team is ecstatic to finally have a definitive structure for telomerase and looks forward to learning more about the intricate assembly process of one of the most complex enzymes in the body: a polymerase as complicated as the ribosome, which reads RNA to produce proteins.