Scientists Tweak Small Molecule Drug to Develop Antiviral against H3N2
Imperceptible to the human eye, the flu virus is one of the most pervasive and persistent on the planet. It mates and spreads in the air. One strain is capable of circumventing the globe in a matter of months.
Annual flu epidemics kill 250,000-500,000 people each year and cause severe illness in 3 million to 5 million. But new strains that jump from animals to humans can be even more devastating if the global population has no immunity to the virus.
This year’s flu rates have yet to reach the levels seen three years ago, but flu season is currently at its peak and current trends suggest they could get worse. This year’s unfolding flu season can be explained threefold, thanks to the most dominant flu strain infecting people—H3N2.
A research team led by the University of California at San Diego has developed a small molecule that they believe could weaken or even stop H3N2 in its tracks. It works by exploiting a flaw in the mechanism the virus uses to replicate, according to the team, which presented their research at the 255th National Meeting & Exposition of the American Chemical Society
(ACS) in New Orleans.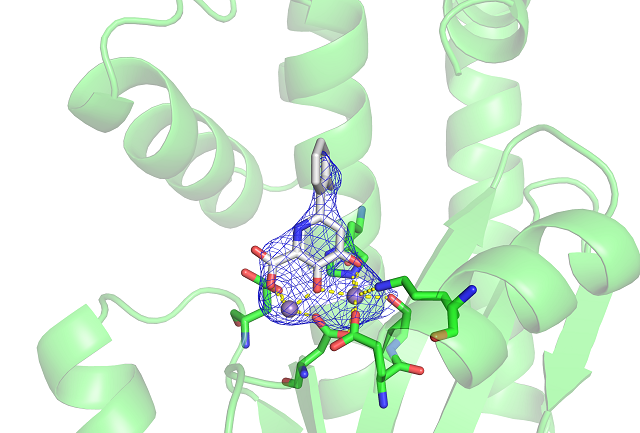
In order to develop an antiviral drug for influenza, scientists had to find an area within its structure that would prove vulnerable. The influenza virus is a lipid-enveloped, negative-sense, single-strand RNA virus, meaning the genetic information it uses for replication is contained in RNA strands held inside a protein shell that is coated by a fatty layer.
Instead of relying on a host’s straightforward DNA replication process as some other viruses do, influenza depends on its own enzyme called RNA-dependent RNA polymerase. So, scientists have consistently focused research efforts on developing a drug that would affect this viral process.
Seth Cohen, who is at the University of California, San Diego and co-founder of Forge Therapeutics, notes that the RNA polymerase complex remains constant across many different versions and mutations of the influenza virus.
Which is why any therapy that target it are not likely to suffer from the issue the vaccine faces; namely, the H3 flaw. The RNA polymerase itself is divided into three subunits. Cohen has homed in on a metal-centered domain within one of the subunits.
Cohen has spent the past two years uncovering how manganese ions bind within the RNA polymerase subunit in order to develop a better drug that would serve as a wrench in the virus’ replication works. “We modified our small-molecule drug so that it would bind to both manganese ions simultaneously,” he says. He then tested the molecule on the RNA polymerase protein. “The modification dramatically improved the potency of the compound over previous drugs we created,” he says. The team is hopeful that in the coming months, it will be just as effective when they challenge the whole influenza virus with the molecule.
“This is a medicinal intervention that will slow down the virus if not completely stop it,” Cohen says. “The drug could potentially eliminate the virus on its own or just sufficiently slow its reproduction so that the body can ultimately clear it. It’s like taking an antibiotic for a viral infection.“






























