3D Printed Patches that Produce Tissue-Saving Vascular Networks
Arterial bypass grafts remain the gold standard for the treatment of end-stage ischaemic disease. Yet patients unable to tolerate the cardiovascular stress of arterial surgery or those with unreconstructable disease would benefit from grafts that are able to induce therapeutic angiogenesis.
Now, using a mouse model of hindlimb ischemia, the researchers identified specific patch patterns that induced growth of organized, tissue-saving blood vessels, demonstrating the potential for the novel technology to address this significant public health problem.
“We know that when growth factors are injected into a tissue, they do induce the sprouting of new blood vessels, but in a disorganized pattern unable to deliver oxygen to ischemic tissues” explained Christopher Chen, M.D., Ph.D., Professor of Biomedical Engineering and Founding Director of the Biological Design Center at Boston University. “Our goal was to use engineering to direct the growth of new vessels into an orderly, functional network.”
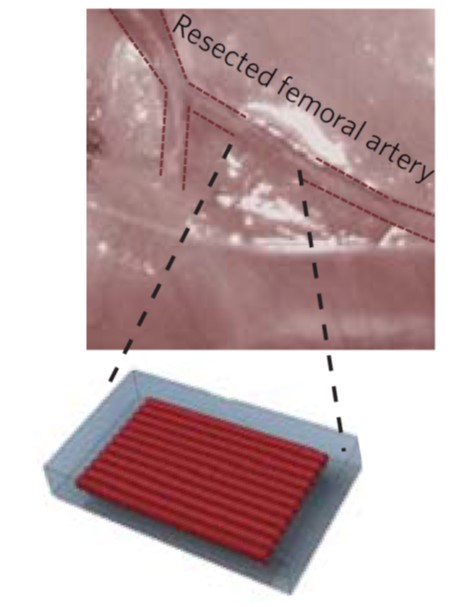
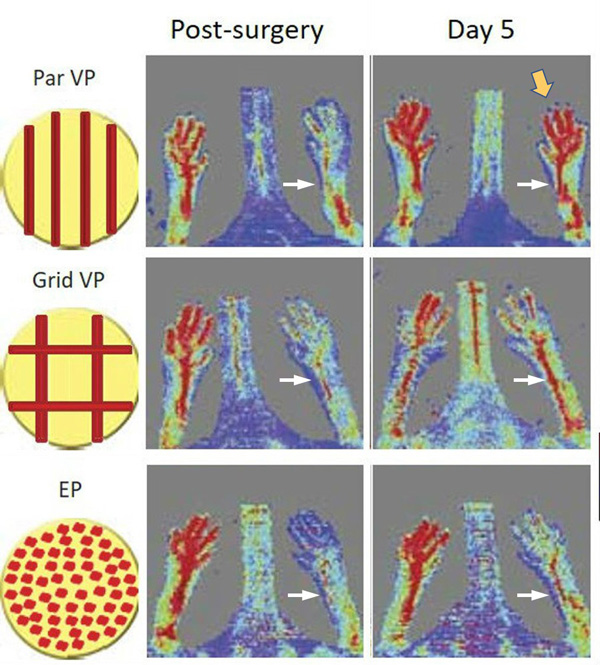
To direct organized vessel formation, the team constructed 3D-printed vascular patches with different patterns of channels. The channels were lined with the endothelial cells that induce the sprouting of new blood vessels. The patterns included straight, parallel rows of channels, a grid pattern with channels crisscrossing each other, and a “no pattern” control with the endothelial cells scattered randomly over the entire patch.
The patch was implanted on animal models with a section of the femoral artery in the back, left leg removed (about a one-centimeter piece). The right leg was left undisturbed for comparison to the treated left leg.
At day five post-surgery the sites with patches bearing straight rows of channels of endothelial cells gave the best result, producing a vigorous network of vessels that oxygenated the foot of the mouse to a level nearly equal to the normal right foot.
“Although we are still at the very early stages of this project, we are encouraged by the initial results,” concludes Chen.
The team intends to continue refining the architectural design of the patches to optimize their effectiveness. The other plan is to continue the collaboration with biologists and clinicians.
“I can’t stress enough how the current and future success of this project is completely dependent on the partnership of engineers, biologists and clinicians,” said Chen. “We are all excited about what can be accomplished when there is so much diverse, yet interdependent expertise focused on beating this major public health problem.”
Adds Rosemarie Hunziker, Ph.D., Director of the NIBIB Program in Tissue Engineering, “The results of this collaboration are an excellent example of how engineers can take what biologists and physicians know about how our bodies work and use the information to create practical, innovative medical treatments.”























