Study Surveys Structure of Enzyme behind DNA Maintenance
Cells often need to make more DNA, for example when they are about to divide or need to repair their genetic information. The building blocks of DNA – also called deoxyribonucleotides – are created through a series of biochemical reactions. Among the enzymes that accomplish these reactions, ribonucleotide reductases (or RNRs, for short) perform a key irreversible step.
RNRs are essential enzymes in all organisms and catalyze the reduction of ribonucleotides into deoxyribonucleotide precursors for replication and repair of DNA. Because RNR is vital for cell proliferation and genome maintenance, drugs that target human RNR are used against some of the most aggressive and challenging to treat cancers, including refractory lymphoblastic leukemia, metastatic ovarian and pancreatic cancers, and melanoma.
Now, researchers at the University Of Colorado School Of Medicine, and the University of Texas Houston Medical Center led by those at MIT have unveiled the structure of this enzyme revealing the likely mechanism for how cells regulate the enzyme.
“People have been trying to figure out whether there is something different enough that you could inhibit bacterial enzymes and not the human version,” says Catherine Drennan, an MIT professor of chemistry and
biology and a Howard Hughes Medical Institute Investigator. “By considering these key enzymes and figuring out what are the differences and similarities, we can see if there’s anything in the bacterial enzyme that could be targeted with small-molecule drugs.”Most of the researchers’ previous work on RNR structure has focused on the version found in E. coli. For those studies, they used X-ray crystallography, a technique that can reveal the atomic and molecular structure of a protein after it has been crystallized.

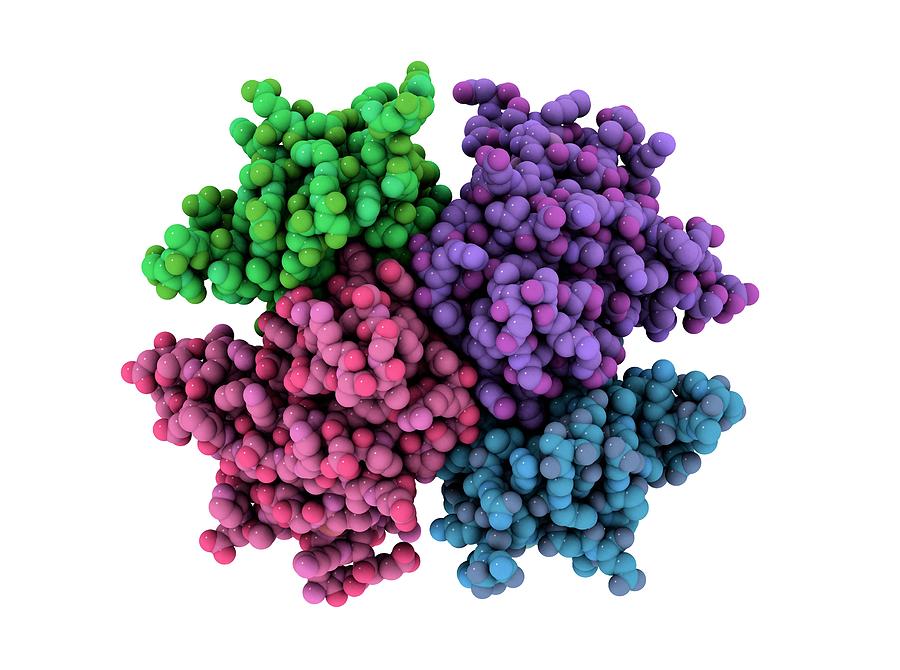
Scientists already knew that RNR consists of two protein subunits known as alpha and beta. In the course of this study, the team used the recent version of the cryo-EM which offered resolutions of about 3 angstroms as opposed to the resolution of about 10 to 20 angstroms it previously offered.
“The technological advances that have allowed cryo-EM to get to such high resolution are really exciting,” Drennan says. “It’s really starting to revolutionize the study of biology.”
The team found found that the human version of the enzyme forms a ring made from six of the alpha subunits. When ATP, which activates RNR, is bound to the enzyme, the ring is unstable and can be easily opened up, allowing the beta subunit to make its way into the ring. This joining of alpha and beta allows the enzyme’s active site, located in the beta subunit, to perform the chemical reactions necessary to produce deoxynucleotides.
However, when the inhibitor dATP is present, the ring becomes much more rigid and does not allow the beta subunit to enter. This prevents the enzyme from catalyzing the production of deoxynucleotides.
This 6-unit ring is not found in the bacterial form of RNR, which instead assembles into a distinct ring containing four alpha subunits and four beta subunits. This means it could be possible to design antibiotics that target the bacterial version but not the human version, Drennan says.
The scientists next intend to investigate the structures of other protein molecules that are difficult to study with X-ray crystallography, including proteins with iron sulfur clusters, which are found in many metabolic pathways.