Re-programmed Skin Cells for Personalized Treatment of MS
Advances in stem cell biology have raised hopes that diseases of the Central Nervous System may be ameliorated by non-hematopoietic stem cell medicines. And Cambridge scientists in their latest study, have, in this direction achieved new heights. Scientists have shown in mice that skin cells re-programmed into brain stem cells, transplanted into the central nervous system, help reduce inflammation and may be able to help repair damage caused by multiple sclerosis (MS).
“Our mouse study suggests that using a patient’s reprogrammed cells could provide a route to personalised treatment of chronic inflammatory diseases, including progressive forms of MS,” says Dr Stefano Pluchino, lead author of the study from the Department of Clinical Neurosciences at the University of Cambridge.
“This is particularly promising as these cells should be more readily obtainable than conventional neural stem cells and would not carry the risk of an adverse immune response.”
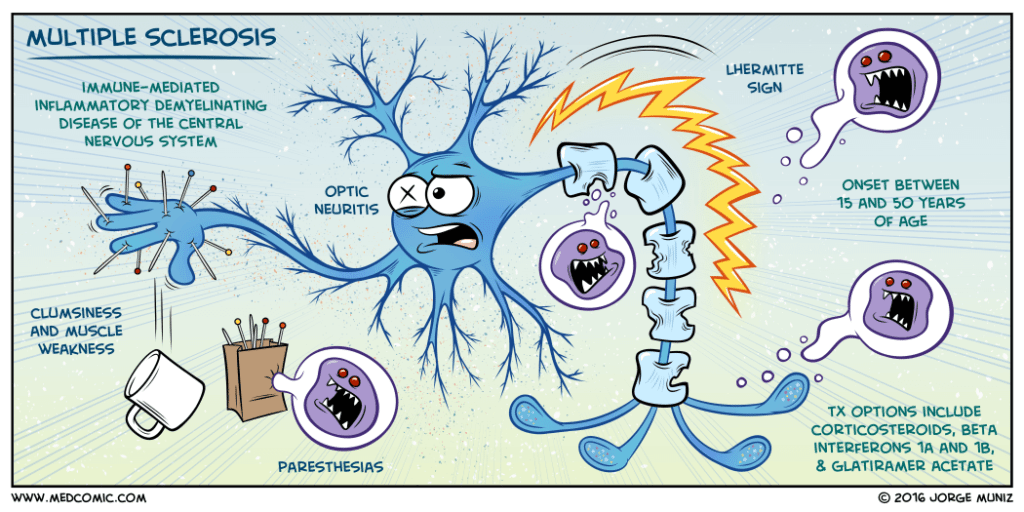
During progressive MS, the immune system harms myelin, the protective sheath around nerve fibers. Thus, it leads to disruption to messages sent around the brain and spinal cord. Symptoms are eccentric and incorporate issues with versatility and adjust torment and serious weariness.
Previous work by the Cambridge team has shown how
Neural Stem Cell transplantation can help to reduce inflammation in animal models of inflammatory central nervous system (CNS) disorders. Therefore, for this latest research, the team hypothesized that the cells may exert therapeutic effects in chronic neuroinflammation by modulating metabolism of MPs and so reduce their inflammatory activities, which would hold back secondary damage to the CNS.They investigated whether NSC and iNSCs (induced neural stem cells) could counteract the metabolic changes in type 1 inflammatory MPs both in vitro and in the experimental autoimmune encephalopmyelitis (EAE) mouse model of MS.
The team observed that injections of either iNSCs of NSCs into the CSF were equally effective at reducing inflammation and damage to nerve axons and myelin. The injected cells induced a specific phenotypic switch of MPs that was associated with reduced levels of succinate in the CSF and reduction in chronic inflammation.
The observation that iNSCs were as effective as somatic NSCs was encouraging. “This is particularly promising as these cells could be more readily obtainable than conventional NSCs and would not carry the risk of an adverse immune response,” Dr. Pluchino states.
“Our work identifies a novel anti-inflammatory mechanism that underpins the regenerative potential of somatic cells and directly induced NSCs, thus paving the way for a new era of personalized stem cell medicines for chronic inflammatory and degenerative neurological diseases.” The team concludes.