“Icebreaker” Molecule Navigates Chromatin Opening Genome for T cell Development
T cell development is orchestrated by transcription factors that regulate the expression of genes initially buried within inaccessible chromatin, but the transcription factors that establish the regulatory landscape of the T cell lineage remain unknown.
But now, researchers at the University of Pennsylvania led by Golnaz Vahedi, Ph.D., an assistant professor of genetics, have been able to plough through the densely condensed, coiled DNA to unlock the secrets of these transcription factors in addition to unveiling how one these factors called TCF-1 enables the expression of previously silent genomic regions.
The new connection between TCF-1 and chromatin will improve our understanding of how T-cell lineages arise. It may also aid the development of therapies that use epigenetic drugs to alter T-cell fate in cancer, autoimmune disorders, and infectious diseases.
The scientists like to think of the transcription factor, TCF-1, as an icebreaker ship that initially opens the ice (condensed, closed chromatin) and keeps a path available for other ships (other transcription factors that work in later stages of development) to steam through the now-open water (unwound chromatin).
TCF-1 ultimately opens chromatin so that DNA can be read to make proteins, and it also keeps chromatin open so that subsequent factors can access DNA to make protein that guide a maturing T cell to its final identity.

“Our lab is interested in understanding how T-cell identity is established,” Vahedi said. “We chose to study T cells because of their all-important role in patrolling the body to clear it of germs and such other dangers as cancer cells.”
The team established the identity-making role for TCF-1 by deleting it in a mouse model and found that most open sites closed, becoming inaccessible in tightly wound chromatin. On the other hand, when they added TCF-1 to a type of common skin cell, it “broke the ice” and opened closed regions by removing chemical groups that tighten chromatin. The elongated fibroblasts reprogrammed to become more T-cell-like in shape and hundreds of T-cell genes were also expressed in these skin cells.
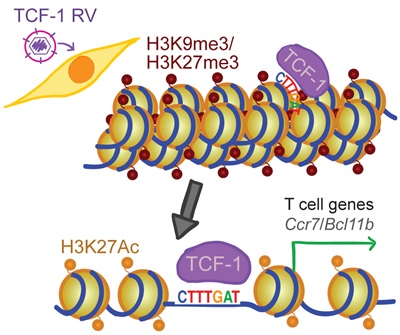
They then re-determined TCF-1 behaviour using computational and epigenomic methods. Attributable to the consistency among many cells, they concluded that TCF-1 control of T-cell fate is fundamentally important in determining what a cell will become.
“We showed how TCF-1 controls T-cell fate through its ability to target closed chromatin and establish the identity of developing T cells,” Vahedi said. “TCF-1 is the focus of many immunology and oncology studies, especially those dealing with checkpoint inhibitors and sick immune cells. We think that the ‘ice-breaking’ ability of TCF-1 can be selectively harnessed to reset ‘old’ T cells to a ‘younger’ state so they can fight invaders again.”