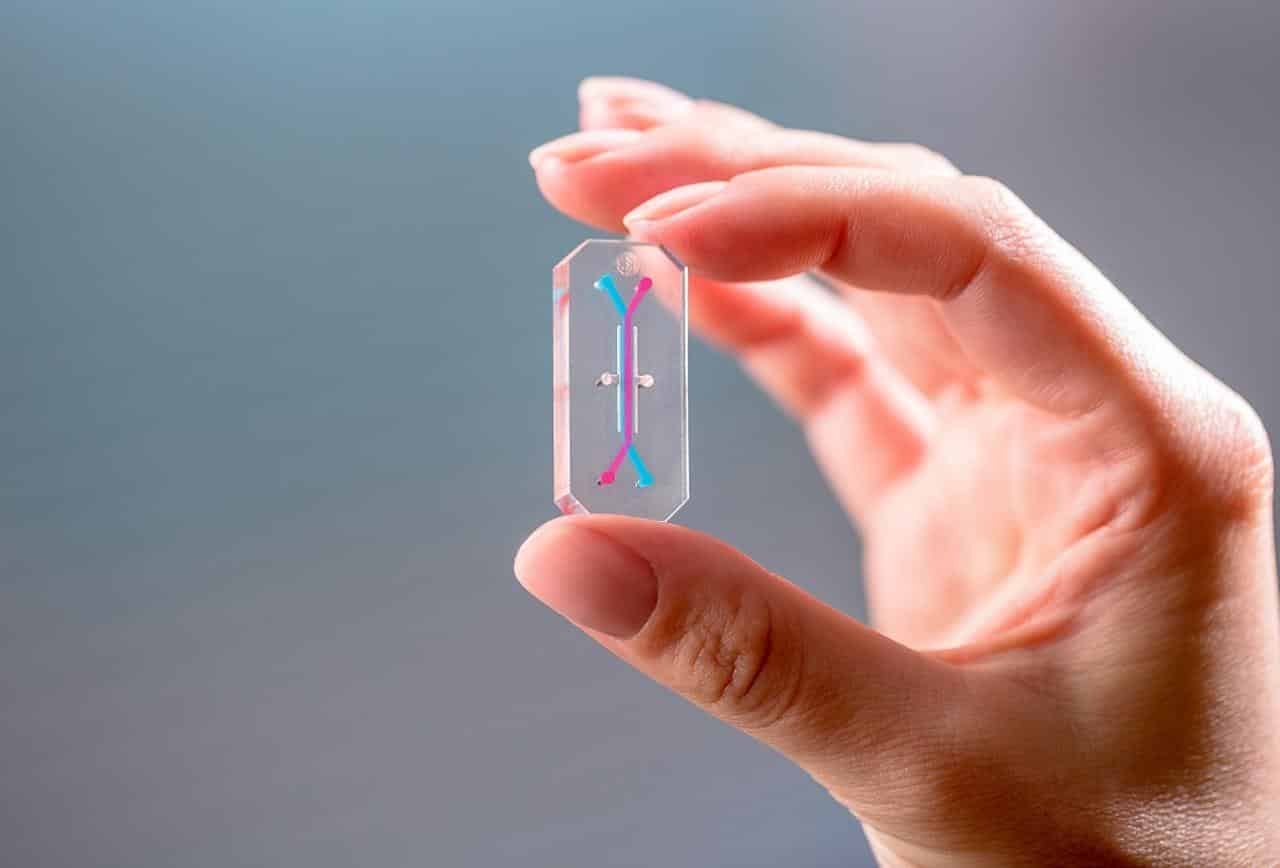
Gut-on-a-Chip Wields Promise for Future of Personalized Medicine
The small intestine is the major site for digestion, drug and nutrient absorption, interaction with commensal microbiome, and development of mucosal immunity, as well as a primary site for many diseases, such as bacterial, viral and parasitic infections and inflammatory bowel disease.
And majorly attributable to the lack of human-relevant responses, many animal models unsuitable to study causal factors and treatment strategies for human intestinal infections and disorders, three-dimensional (3D) human tissue surrogates, such as intestinal organoids (also known as enteroids) have emerged as promising alternatives.
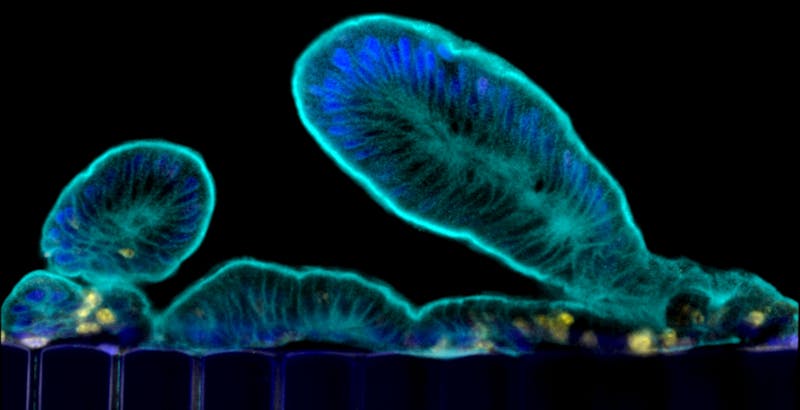
Organoids faithfully recapitulate the cellular diversity of the intestinal epithelium and are ideally suited for in situ visualization and continuous monitoring of epithelial development and differentiation. But the catch is, these organoids form all of the cell types present in human intestine, but they grow as cysts surrounded by thick extracellular matrix gels with their “apical” cell surface (which is normally exposed to the content of the gut) facing an enclosed lumen. This prevents the study of dynamic processes involving the intestinal barrier, including nutrient and drug transport, as well as its interactions with the microbiome.
Therefore, researchers at the Wyss Institute led by Founding Director, Donald Ingber
, M.D., Ph.D., had previously engineered a microfluidic “Organ-on-a-Chip” (Organ Chip) culture device in which cells from a human intestinal cell line originally isolated from a tumor were cultured in one of two parallel running channels, separated by a porous matrix-coated membrane from human blood vessel-derived endothelial cells in the adjacent channel.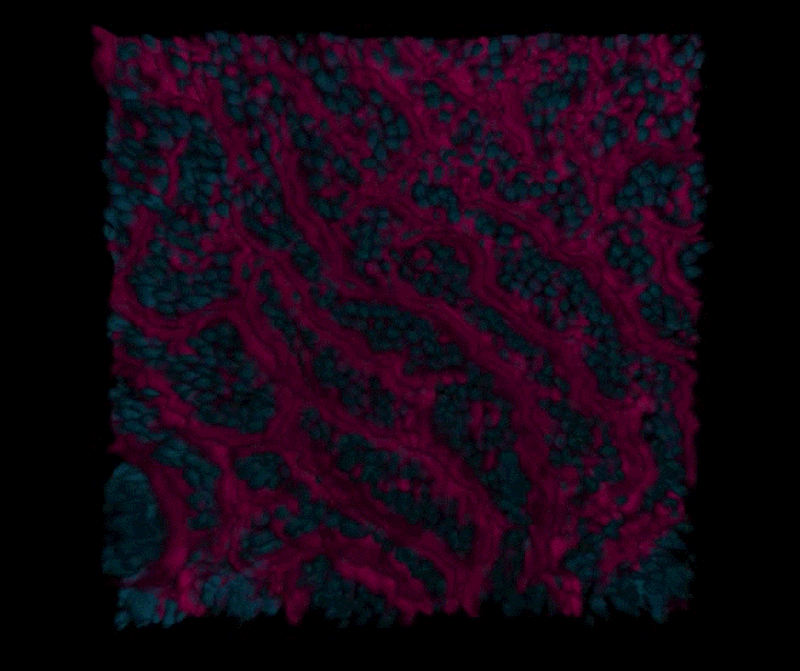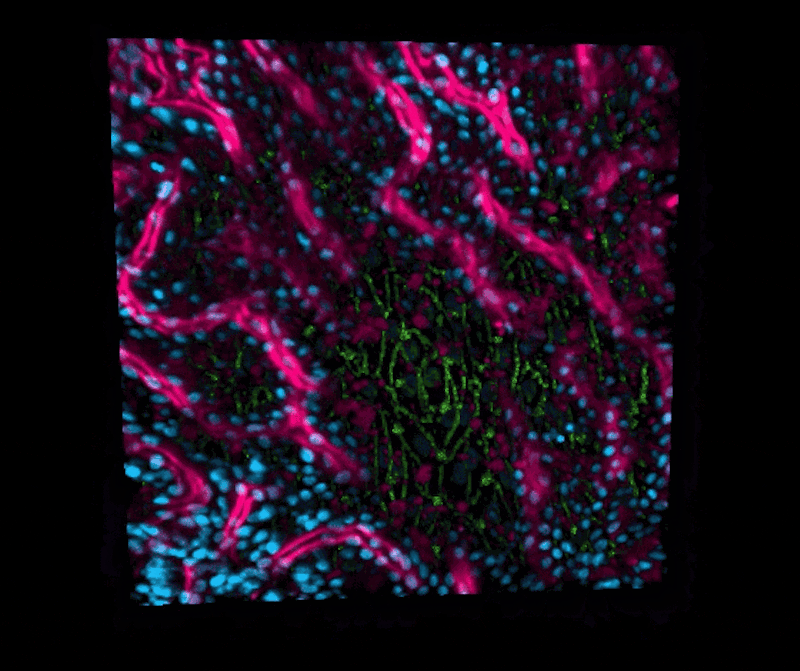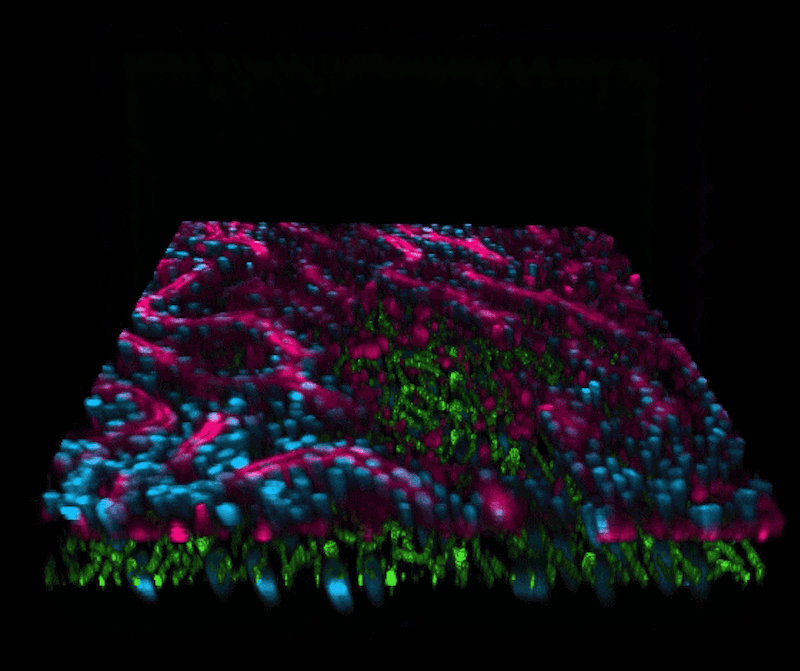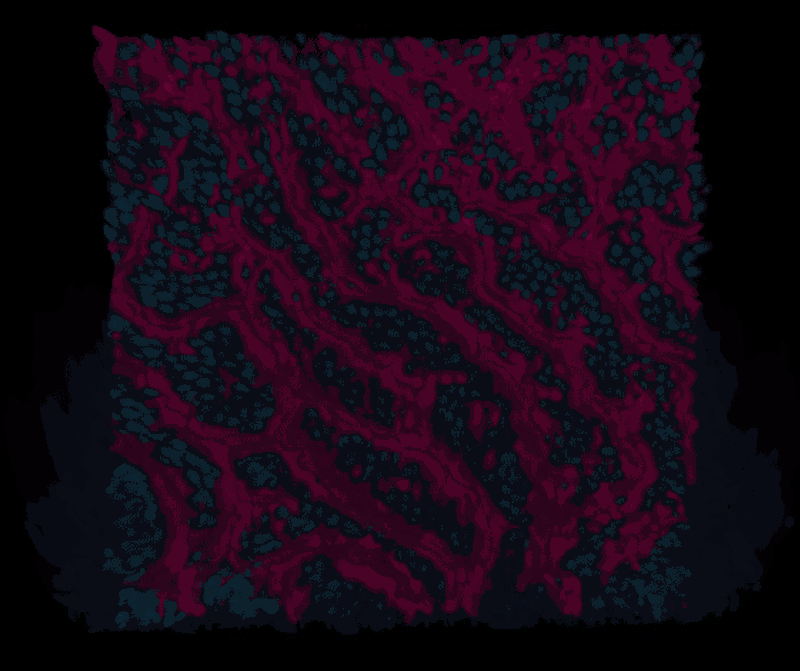
Admittedly, the chip enabled new insights into how flow and cyclic peristalsis affects intestinal differentiation and function, but it could not be used to study processes that relied on normal intestinal cells from individual donors.
“We are now able to leverage the organoid approach to isolate intestinal stem cells from human biopsies, but we break up the organoids and culture the patient-specific cells within our Organ Chips where they spontaneously form intestinal villi oriented towards the channel lumen, and the epithelium in close apposition to human intestinal microvascular endothelium,” said Ingber, who is also the Judah Folkman Professor of Vascular Biology at Harvard Medical School (HMS) and the Vascular Biology Program at Boston Children’s Hospital, as well as Professor of Bioengineering at Harvard’s John A. Paulson School of Engineering and Applied Sciences (SEAS). “This approach presents a new stepping stone for the investigation of normal and disease-related processes in a highly personalized manner, including the transport of nutrients, digestion, different intestinal disorders, and intestinal interactions with commensal microbes as well as pathogens.”
In the course of this study, researchers used a microfluidic device composed of a clear, flexible polymer containing parallel microchannels separated by a porous extracellular matrix membrane. One channel is coated with human intestinal epithelial cells and the other with human endothelial cells which mimic the blood vessel wall.
This chip was then exposed the chip to a radiation dose of 8 Gray, which is known to cause gastrointestinal effects in humans. The team then observed increases in several markers of cell damage in both the endothelial and epithelial cells: cell death, generation of reactive oxygen species, double-stranded DNA breaks, degradation of lipids in the cell membrane, and loss of microvilli structure, as well as junction disruptions between neighbouring cells and of the mucous membrane that protects the intestinal wall.
Interestingly, the endothelial cells in the blood vessel channel had a stronger response to radiation than the epithelial cells in the intestinal channel in terms of ROS generation, lipid degradation, and DNA fragmentation. Furthermore, the endothelial cells experienced peak apoptosis levels about 24 hours after exposure, while epithelial cells were hardest hit at 48 hours, suggesting that the endothelium is more sensitive to radiation.
When the scientists repeated the experiment on a chip that only contained epithelial cells, they observed that the irradiated cells did not have the typical response to radiation: their microvilli were not blunted, their mucosal barrier was not disrupted, their cell barriers remained functional, and they had lower ROS generation. This surprising result suggests that endothelial cells play a key role in the gastrointestinal damage observed in radiation injury.
“This finding helps explain why other models of the human gut that don’t include endothelial cells generally fail to mimic the gut’s response to radiation injury,” says Oren Levy, Ph.D., M.B.A., co-author of the paper and Staff Scientist at the Wyss Institute.
“More studies are needed to confirm the link between endothelial and epithelial cell responses, but we think that ROS generated by endothelial cell damage will prove to be the driving force behind epithelial cell damage, and this could serve as a target for future anti-radiation therapeutics.”
“In the future, such efforts could allow us to much better understand human-microbiome interactions, model malnutrition disorders and inflammatory diseases of the gut, and perform personalized drug testing,” said co-first author Alessio Tovaglieri, a Graduate Student at the Department of Health Science and Technology at ETH Zurich in Switzerland, who performs his thesis work on Ingber’s team.