Scientists Develop Blood-Vessel-On-A-Chip to Model Coagulation In Vitro, Pave Way for New Anti-Inflammatory Drug Candidate
Best known for its anticoagulant activity, Activated protein C (APC), a vitamin K-dependent serine protease, is generated from thrombin-mediated cleavage of circulating protein C (PC). It has been used medically to treat severe blood infections and wounds; however, its use is limited because it inhibits thrombin and impacts the ability of the blood to clot which increases bleeding risk.
The processes of inflammation and coagulation have been found to overlap, with the presence of inflammatory mediators being shown to modulate the activity of natural anticoagulant mechanisms and vice versa. Inflammation is one of the most key and fraught processes in the human body but it also can increase thrombin production, which can lead to dangerous blood clots and other conditions.
Now, a collaborative team of researchers from the Division of Hemostasis and Thrombosis at Beth Israel Deaconess Medical Center (BIDMC) and the Wyss Institute at Harvard University have discovered that synthetic APC-mimicking small molecules called “parmodulins” provide anti-inflammatory and anti-thrombotic protection to endothelial cells on par with APC’s without interfering with normal blood clotting and coagulation, making them attractive new drug candidates.
This work was enabled by leveraging the Wyss Institute’s Organ-on-a-Chip technology to model thrombosis within a human blood vessel in vitro.
“We essentially performed a mini pre-clinical trial of parmodulins’ effect on the endothelium, and not only determined the pathway through which parmodulins function, but also demonstrated that they help protect endothelial cells from inflammatory damage,” says former Wyss postdoc Abhishek Jain, Ph.D., who is now an Assistant Professor and director of the Bioinspired Translational Microsystems lab at Texas A&M University.
To evaluate the activity of parmodulins on endothelium, Karen De Ceunynck, Ph.D., postdoctoral research fellow at BIDMC, incubated human endothelial cells with parmodulin 2 in vitro for 4 hours and then exposed them to the thrombin-inducing inflammatory agents lipopolysaccharide (LPS) or tumor necrosis factor-α (TNF-α).
In the parmodulin-exposed cells, both agents’ ability to generate thrombin was reduced by over 50% compared with non-parmodulin-exposed cells. However, parmodulin 2 did not inhibit the activity of factor V or factor X, proteins that function in blood coagulation.
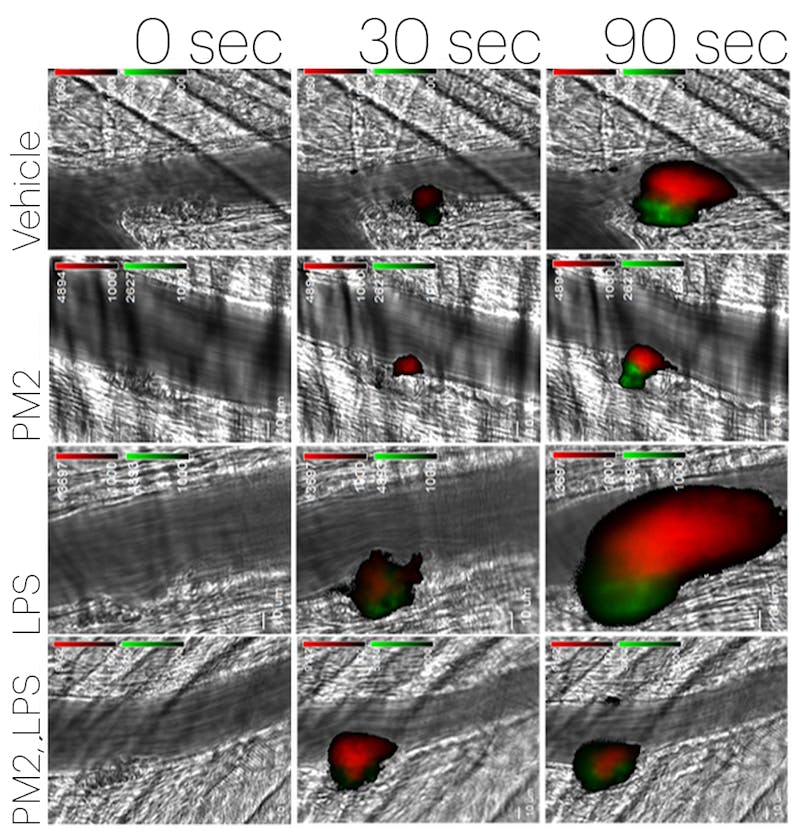
The team later used a Wyss-developed blood-vessel-on-a-chip in order to confirm this.
The blood-vessel-on-a-chip consists of microfluidic channels embedded in a clear polymer chip, coated with collagen, and lined by human endothelial cells. Whole blood was perfused through the chip to simulate the flow conditions within human blood vessels, to which were added different pro- and anti-inflammatory compounds to evaluate the response of the endothelium.
Results indicated that parmodulin exposure blocks the thrombotic response of endothelium to inflammatory stimuli without affecting blood coagulation in humans – a significant improvement over APC.

“We observed that the cytoprotective response induced by parmodulin 2 happened very quickly, and confirmed its rapid onset in time course and gene expression assays,” says Peters.
Further, in vivo studies in mice showed that parmodulin 2 reduces the binding of white blood cells to blood vessels and impairs platelet and fibrin accumulation at injury sites during the inflammatory response, confirming the anti-thrombotic and anti-coagulant activity of parmodulin 2 observed in vitro. Additionally, parmodulins do not interact with many of APC’s other binding partners, making it much more targeted to PAR1 and reducing other side effects.
“The discovery of an anti-inflammatory molecule that prevents endothelial thrombosis but also preserves normal blood coagulation is a major step toward an alternative and better approach to treating inflammatory disease,” says Rob Flaumenhaft, M.D., Ph.D., Professor of Medicine at Harvard Medical School, Chief of the Division of Hemostasis and Thrombosis at BIDMC, and corresponding author of the paper. “Furthermore, nearly all other pharmaceuticals that target transmembrane PAR1-like receptors bind to the exterior side of the receptor; parmodulin 2 represents a paradigm shift for compounds targeting these receptors because it acts on the cellular side of the protein. We are excited to see if we can advance it to clinical trials.”
“This work provides another example of how organ-on-a-chip technology can enable faster and safer development and evaluation of drugs that could help patients around the world,” says co-author and Wyss Institute Founding Director Donald Ingber, M.D., Ph.D., who is also the Judah Folkman Professor of Vascular Biology at HMS and the Vascular Biology Program at Boston Children’s Hospital, as well as Professor of Bioengineering at Harvard’s John A. Paulson School of Engineering and Applied Sciences (SEAS).