Autoantibodies That Attack ‘Self’ Tissues into Anti-Inflammatory Antibodies
Autoantibodies are antibodies that react with self-antigens. These antigens may be found in all cell types (e.g. chromatin, centromeres) or be highly specific for a specific cell type in one organ of the body (e.g. thyroglobulin in cells of the thyroid gland). For this very reason, many autoantibodies are useful biomarkers of disease.
The presence of autoantibodies highly specific for target organs in organ-specific autoimmune diseases, such as thyroiditis, type 1 diabetes mellitus and primary biliary cirrhosis, strongly suggest that autoantibodies are stimulated by inflammation in the target organ.
Tissue injury in autoimmune disease results because the self antigen is an intrinsic component of the body and, consequently, the effector mechanisms of the immune system are directed at the body’s own tissues. Also, because the adaptive immune response is incapable of removing the offending autoantigen from the body, the immune response persists, and there is a constant supply of new autoantigen, which amplifies the response.
Now, a team of Massachusetts General Hospital (MGH) investigators has found a way to engineer antibodies within an organism, converting autoantibodies that attack “self” tissues into anti-inflammatory antibodies in animal models of two autoimmune diseases.
“We were able to convert antibodies that cause autoimmune disease into anti-inflammatory antibodies by specifically modifying the sugars attached to the antibodies,
” says Robert Anthony, PhD, of the Center for Immunology and Inflammatory Diseases in the MGH Division of Rheumatology, Allergy and Immunology, who led the study. “While more work is required, we hope that this approach of anti-inflammatory antibody conversion will have a beneficial effect on patients suffering from autoimmune and inflammatory diseases.”IgG antibodies are key players in the immune system and are essential for clearing pathogenic microbes by acting as a bridge between adaptive and innate immune system pathways, however, these antibodies (IgM and, occasionally, low titer IgG autoantibodies) are often detected in healthy individuals.
Further, Intravenous immunoglobulin (IVIG), at high dosage levels can suppress, rather than enhance the immune response and is used to treat several inflammatory and autoimmune conditions. Whether activated antibodies stimulate an inflammatory or anti-inflammatory response appears to be determined by specific sugar molecules called glycans attached to the Fc region — the tail-like section of a Y-shaped antibody.
In their previous outing, the investigators of the study found that the anti-inflammatory effect of high-dose IVIG can be attributed to a minor fraction of antibodies to which a glycan called sialic acid has been attached to their Fc region.
While glycans are usually attached to the proteins making up antibodies while they are being assembled within cells, recent evidence suggests they could be modified outside of the cellular environment.
Therefore the team set out to determine whether administration of a transferase, an enzyme that moves chemical groups like glycans from one molecule to another, could convert inflammatory antibodies to anti-inflammatory forms within a living animal.
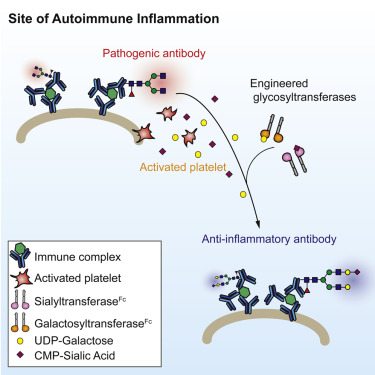
Since the attachment of sialic acid to an Fc group requires the presence of another glycan called galactose, the team created two enzymes termed B4Fc and ST6Fc, which induce the attachment of galactose and sialic acid respectively.
Although intravenous administration of either enzyme alone did not reduce inflammation in a mouse model of rheumatoid arthritis, simultaneous administration of both in a form called B4ST6Fc had anti-inflammatory effects similar to those of high-dose IVIG. B4ST6Fcadministration also reduced kidney damage in a mouse model of lupus-related kidney inflammation.
Additional experiments found little evidence that enzyme administration affected antibodies not involved with the specific autoimmune condition and also showed that platelets, which become activated at the site of antibody-driven inflammation, provided the sialic acid and galactose attached to the Fc region by the B4ST6Fc enzyme.
Mr. Anthony explains that, while IVIG can be effective against many inflammatory and autoimmune diseases, it is in short supply, the treatment is expensive, and administration is time consuming. “We found that our enzymes were effective at a 400-fold lower dose than high-dose IVIG, and by manipulating the enzymes already in an organism, our method eliminates the need for a lengthy IVIG infusion.” His team will be exploring questions in future studies such as appropriate dosage, optimal administration and whether repeat treatments would be required.