Multifunctional Protein found to be Critical for Blood Cell Development
Nuclear and cytoplasmic compartments of interphase eukaryotic cell are separated by the nuclear envelope (NE), which is formed from two closely juxtaposed membranes, the outer nuclear membrane (ONM) and the inner nuclear membrane (INM), respectively.
The ONM is continuous with the rough endoplasmic reticulum, whereas the INM contains unique transmembrane proteins, which establish contacts with chromatin and the nuclear lamina. Large multi-protein complexes known as nuclear pore complexes (NPCs) perforated the NE to allow molecular trafficking between the cytoplasm and the nucleus. The NPC is composed of approximately 30 different proteins, termed nucleoporins (Nups), which are broadly conserved between yeast, vertebrates and plants.
Recent studies have shown that a subset of nucleoporins can detach from the NPC and move into the nuclear interior to regulate transcription. One such nucleoporin is Nup98.
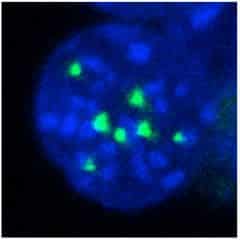
In a new finding, a team of researchers from the Salk Institute has uncovered a previously unknown job for the nucleoporin-Nup98 in mouse cells. In addition to helping control the movement of molecules in and out of the nucleus of the cell, they found that it also helps direct the blood cells development, enabling immature blood stem cells to differentiate into many specialized mature cell types. Further, they discovered the mechanism by which—when this differentiation process is disturbed can contribute to the formation of certain types of leukemia.
Till date, the mechanism of how nup98 regulates transcription was unknown. The investigators found that it acts through a link with a protein complex called Wdr82-Set1/COMPASS, which is part of the cell’s epigenetic machinery.
“This epigenetic process helps to control when genes are transcribed into proteins and when transcription is blocked,” says Martin Hetzer, Salk’s Chief Science Officer and the study’s senior author, who also holds the Jesse and Caryl Phillips Foundation Chair.
“This is the first mechanistic insight of how one of these nup proteins works in mammals,” Hetzer adds. “We have only touched the surface here in uncovering how this evolutionarily conserved mechanism works in mammalian cells.” Future work in his lab will extend the study of nup98 to primates and humans.
While Hetzer has no immediate plans to pursue their findings as an avenue for developing leukemia drugs, he says it’s likely that others may pick up on this aspect of the research.