Cellular Debris-Removal System Might be Fuelling Tumours
One of the mechanisms that the body uses to remove dying/dead cells is called efferocytosis (“to carry to the grave”). In efferocytosis, dying/dead cells are engulfed by phagocytic cells such as macrophages and dendritic cells. Generally, efferocytosis plays essential roles in tissue homeostasis, embryologic development, immunity, and resolution of inflammation.
However, a new study found that efferocytosis of dying cancer cells may actually accelerate tumor growth.
The most common site of prostate cancer metastasis is bone, with an incidence of 65%–80% in patients with advanced disease. Once cancer cells spread to bone, they significantly alter normal bone remodeling, resulting in bone fractures, nerve compression, pain, and hypercalcemia. In bone, cancer cells find a protective and supportive microenvironment that promotes metastatic outgrowth. The bone microenvironment is rich in factors that promote the mobilization, proliferation, and differentiation of proinflammatory cells, which interact with disseminated tumor cells, promoting survival and colonization.
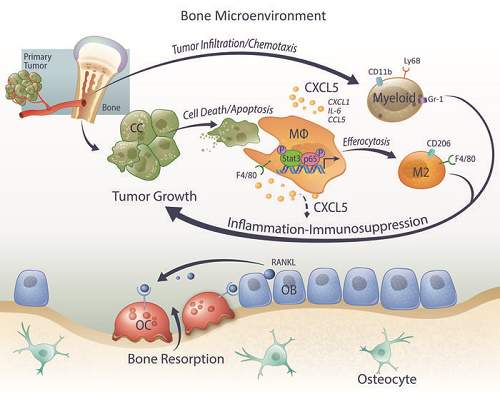
Persistent inflammation also exacerbates cell stress and tissue damage, causing apoptotic/necrotic cell death. The clearance of dying cells occurs mainly through phagocytosis by macrophages and other cells. Phagocytosis of apoptotic cancer cells aka efferocytosis
is a process often underestimated during tumor progression due to rapid clearance by phagocytes. Tumor cell death, significantly amplified by chemotherapies or other targeted therapies, triggers extensive efferocytosis, which has been suggested to accelerate further tumor growth at least in part by inducing M2-like macrophage polarization and resultant protumoral functions.Therefore, the researchers at the University of Michigan used in vivo models to investigate the skeletal tumor growth and apoptosis-inducible prostate cancer cells, and the role of apoptotic cancer cell clearance in skeletal tumor progression.
The study found that with metastatic prostate cancer cells, efferocytosis produced a pro-inflammatory protein called CXCL5 that isn’t normally released during cellular cleanup in healthy situations. Researchers found this CXCL5 protein stimulated tumor growth.
When researchers induced cell death in mouse bone tumors, it correlated with an increase of CXCL5, and the growth of tumors with induced cell death accelerated. Blocking the CXCL5 protein in mice, however, hindered tumor progression.
“In the presence of cancer, uncontrolled cell growth is also accompanied by a high, or significant, amount of cancer cell death,” and those dead cells must be removed, says study lead author Hernan Roca, associate research scientist at the University of Michigan School of Dentistry. “The challenge for the future is to understand how to treat these patients to avoid this pro-inflammatory and tumor promoting response, while still preserving the essential function of cell removal.”
The researchers also analyzed blood samples from bone-metastatic prostate cancer patients, localized prostate cancer patients, and normal controls. The results showed that patients with bone metastasis of prostate cancer had higher levels of CXCL5 in their blood.
Collectively, these data suggest that efferocytosis of dying cancer cells increases expression of proinflammatory cytokines like CXCL5 to facilitate prostate tumor metastases in bone. Therefore, CXCL5 could be targeted to treat prostate cancer. More research is required to study the role of efferocytosis in other types of cancer.