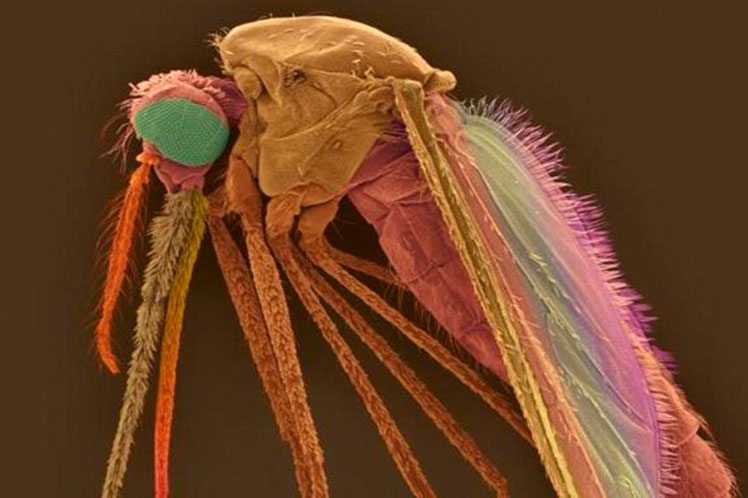
Scientists Design CRISPR edited, Self-Destructible Transgenic Mosquitoes
Yellow, Three-Eyed, Wingless Mosquitoes Created Using CRISPR Gene Editing Tool with the Cas9 enzyme embedded in their germline.
The development of CRISPR/Cas9 technologies has dramatically increased the accessibility and efficiency of genome editing in many organisms. In general, in vivo germline expression of Cas9 results in substantially higher activity than embryonic injection. However, no transgenic lines expressing Cas9 have been developed for the major mosquito disease vector Aedes aegypti.
The yellow fever mosquito, Aedes aegypti, is the principal vector of many arboviruses, such as dengue, chikungunya, yellow fever, and zika. These pathogens are globally widespread and pose significant epidemiological burdens on infected populations, resulting in hundreds of millions of infections and over 50,000 deaths per year. Due to the hazards they impose, many methods for controlling Ae. aegypti populations have been implemented, with the most common being chemical insecticides.
However, chemical control has proven incapable of stopping the spread of Ae. aegypti, primarily due to the mosquito’s ability to rapidly adapt to new climates, tendency to oviposit in minimal water sources, desiccation-tolerant eggs, and quick development of insecticide resistance.
Therefore, significant efforts are currently underway to discern the underlying molecular and genetic mechanisms
important for arboviral vector competence, with the overall aim of developing insecticide-free ways to disrupt viral disease cycles.Joining the league, researchers at the University of California, Riverside have developed transgenic mosquitoes that stably express the Cas9 enzyme in their germline. The addition of Cas9 will enable the use of the CRISPR gene editing tool to make efficient, targeted changes to the mosquitoes’ DNA.

As proof of concept, the researchers used the system to disrupt cuticle, wing, and eye development, producing completely yellow, three-eyed and wingless mosquitoes. Their long-term goal is to use Cas9-expressing mosquitoes together with another technology—called gene drives—to insert and spread genes that suppress the insects while avoiding the resistance that evolution would typically favor. Aedes aegypti are major carriers of dengue, chikungunya, yellow fever, and Zika viruses, and are rapidly becoming resistant to commonly used pesticides.
This is just a first step, however, according to lead researcher Omar Akbari, an assistant professor of entomology at UC Riverside, who published the study. Ultimately, the plan is to combine CRISPR-Cas9 with the use of gene drives systems, a technology that increases the chance for a particular gene to express from a parent organism to its offspring.
“These Cas9 strains can be used to develop split-gene drives which are a form of gene-drive by which the Cas9 and the guide RNA’s are inserted at separate genomic loci and depend on each other for a spread. This is the safest way to develop and test gene drives in the laboratory to ensure no spread into the wild,” Akbari said.
The team is aiming at using Cas9-expressing mosquitoes together with another technology called gene drives. This will cause the mutations to spread and also avoid the resistance to reproduction that the mosquito will display toward mutated mosquitoes that evolution would typically favor.
Gene drives significantly raise the odds, from 50% to 99%, that a gene or set of genes will be transmitted to offspring. This count can possibly increase to 100% when a target gene is subjected to disruption in multiple sites, an approach known as multiplexing that has recently been mathematically modeled by researchers.
Gene drives can be involved to bias genetic inheritance in favor of genes that are self-destructive and spread rapidly, similar to those that disrupt fertility, and could be a cost-effective and environment-friendly approach to control the populations of disease-spreading insects.
However, using gene drives does bring with it some unwanted effects. One of the most concerning thoughts is that a gene drive could obtain the power to effect an entire species. Therefore, the gene drives should be dealt with great thought and educated hesitation. The dangers don’t seem to concern Abkari, he concluded, “Next steps should be undertaken to identify the regulatory sequences that can be used to express the guide RNAs from the genome, and once these sequences are identified gene drives in the species should be turnkey.”