Minimising Aggregation of Gritty Blobs of RNA ,Help Challenge Alzheimer
Cytoplasmic RNA granules in germ cells (polar and germinal granules), somatic cells (stress granules and processing bodies), and neurons (neuronal granules) have emerged as important players in various aspects of cell biology as well as medicine.
So-called heat stress granules (SGs) contain mRNAs encoding most cellular proteins but exclude mRNAs encoding heat shock proteins. Compositionally similar SGs appear in the cytoplasm of mammalian cells exposed to environmental stress (e.g., heat, oxidative conditions, UV irradiation, and hypoxia). In response to stress, eukaryotic cells reprogram mRNA metabolism to repair stress-induced damage and adapt to changed conditions.
During this process, the translation of mRNAs encoding “housekeeping” proteins is aborted, whereas the translation of mRNAs encoding molecular chaperones and enzymes involved in damage repair is enhanced. Selective recruitment of specific mRNA transcripts into SGs is thought to regulate their stability and translation.
Scientists now know that the neurodegenerative disease is caused by gradual death of brain cells.
While a cure is still to be found, it is thought clearing neurodegenerative disease-causing plaques and tangles from the brain could be the answer – and our exposure to viruses and toxins may have something to do with it.
A new study by Boston University in the United States has found that reducing levels of ‘stress granules’ may help fight the disease, and the discovery has been hailed as a “new chapter” in Alzheimer’s research.
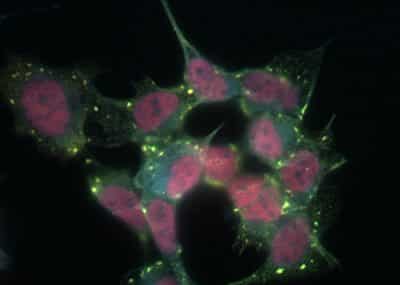
A feature of neurodegenerative disease is the accumulation of insoluble protein aggregates in the brain. In some conditions, including Amyotrophic Lateral Sclerosis and Frontotemporal lobar degeneration, the primary aggregating entities are RNA binding proteins.
Through regulated prion-like assembly, RNA binding proteins serve many functions in RNA metabolism that are essential for the healthy maintenance of cells of the central nervous system. Those RNA binding proteins that are the core nucleating factors of Stress Granules (SGs), including TIA-1, TIAR, TTP and G3BP1, are also found in the pathological lesions of other neurological conditions, such as Alzheimer’s disease, where the hallmark aggregating protein is not an RNA binding protein, this new study has found.
The researchers, led by Benjamin Wolozin at the Boston University School of Medicine, US, had previously shown that when pathological tau accumulates in a brain cell culture, it goes hand in hand with another change.

There is also proliferation of so-called “stress granules”, tiny bundles of proteins that appear in cells stressed by things as diverse as viruses, toxins, temperature change and starvation.
But the researchers were curious. Could TIA1 and the stress granules be part of the problem, contributing to the disease itself?
To find out they enlisted transgenic mice, bespoke bred to over-produce tau and display the animal equivalent of Alzheimer’s disease. The researchers then added another tweak. Some of those mice were also genetically modified to make less TIA1; around 50% less at three months of age.
That modification produced some startling findings.
“This is a pretty big new chapter in the study of Alzheimer’s disease,” says Wolozin, senior author on the paper, whose work is funded by the National Institutes of Health, the Alzheimer’s Association, the Cure Alzheimer’s Fund, and others. “Amyloid is still toxic. Tau is still toxic. Those are still important targets. But there’s this whole other pathway that you can really go in and start drugging.”
When a cell gets stressed out, Wolozin explains, it can act just like a person—hunkering down, stopping all unnecessary activities, and waiting for the storm to pass. “The cell says, ‘uh-oh, I’m not going to worry about being a great person right now,’” he says. “‘I’m just going to deal with this stress.’” For a cell, this means blocking RNA segments that are busily doing the non-essential, daily work of the cell. Molecules called RNA-binding proteins scurry out of the nucleus, round up the RNA into stress granules for safekeeping, and then, when the stress ends, release them to get back to business.
The first batch of mice which were engineered to express a mutation in the tau gene were witnessed to be demonstrating the accumulation of tau after about three months, after about six months, they start to show severe behavioral problems; and by nine months, they nearly all die.
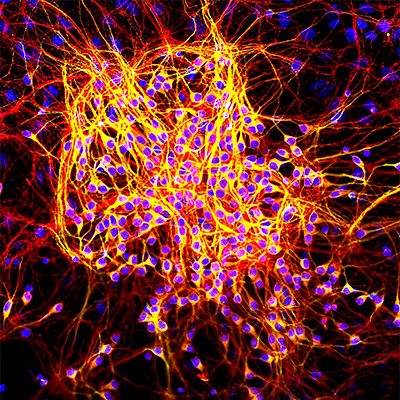
The scientists then bred a group of these mice to be born with only one copy of the gene for TIA1, instead of the normal two. They watched all the mice grow, and what happened next was striking. On schedule, the tau mice started to show signs of brain atrophy: limb weakness, hunched posture, and worsening performance on behavior and memory tests. By contrast, the mice with one copy of the TIA1 gene knocked out showed no signs of mental or physical decline at six months and lived 21.5 percent longer than their counterparts.
“These mice still are affected by disease and die prematurely,” says Dan Apicco (MED’17), first author on the paper and now a postdoctoral scientist at Biogen. “The process just happens much less rapidly.”
When the scientists autopsied the mouse brains, they found another surprise. The brains showed protected nerve cells and a healthy cortex, a far cry from the atrophied brains of their counterparts. But weirdly, the healthy brains showed far more tangles of tau.
The beneficial effects of reduced TIA1 were associated with an increase in the number of tau tangles, the defining feature of Alzheimer’s disease. Wolozin explains how this could be so:
“Reducing TIA1 shifted tau accumulation from small to large clumps, decreasing the amount of small tau clumps and producing a proportional increase in the large tau clumps that generate neurofibrillary tangles and are less toxic,” he says.
In the study of tau toxicity, then, it would seem that size really does matter.























