Researcher Developing Antidotes to Neutralize Chemical Weapons
Among the Weapons of Mass Destruction (WMD), chemical warfare (CW) is probably one of the most brutal created by mankind. CW agents are extremely toxic synthetic chemicals that can be dispersed in a gas, liquid or aerosol or as agents adsorbed to particles to become a powder. These CW agents have either lethal or incapacitating effects on humans.
Also, other toxic agents such as pesticides among others pose a serious health threat worldwide.
Whether released deliberately or emitted by accident from industrial chemical operations during processing, shipping, or storage, they wreak havoc on the natural ecosystem.
The toxic chemicals that have been used as chemical weapons, or have been developed for use as chemical weapons, can be categorized as choking, blister, blood, or nerve agents. The most well-known agents are as follows: choking agents—chlorine and phosgene, blister agents (or vesicants)—mustard and lewisite, blood agents—hydrogen cyanide, nerve agents—sarin, soman, VX.
Of course, some toxic chemicals, and/or their precursors, are utilized globally in industry. For example, toxic chemicals are employed as basic raw material, or as anti-neoplastic agents, which prevent the multiplication of cells, or as fumigants, herbicides or insecticides. Such chemicals are considered chemical
weapons if they are produced and stockpiled in amounts that exceed requirements for those purposes that are not prohibited under the Convention.Now, Jin Montclare, an associate professor in the Department of Chemical and Biomolecular Engineering at the NYU Tandon School of Engineering is trying to thwart these agents by improving upon compounds known to neutralize them.
Montclare is working in a U.S. government program to do so and is awarded a grant by the U.S. Government’s CounterACT (Countermeasures Against Chemical Threats) program under the National Institutes of Health, which supports research to prevent and treat exposure.
The $349,040 grant from the government furthers Montclare’s work on phosphotriesterase (PTE), a compound that can deactivate the neurotoxic agent organophosphorus, the active compound in a number of pesticides and a rogue’s gallery of chemical warfare agents like VX, the most toxic nerve agent ever created that is considered a weapon of mass destruction.
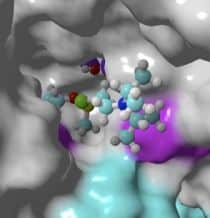
Her previous work had focussed on phosphotriesterases, which have the unique capability of degrading chemicals in a class known as organophosphates, which are found in everything from industrial pesticides to the sarin gas used in chemical warfare. Organophosphates permanently bond to neurotransmitters in the brain, interfering with their ability to function and causing irreversible damage. The ability of phosphotriesterases to detoxify organophosphates has been previously documented; however, applications using the protein for this purpose have been limited by its short half-life and instability at high temperatures. The study involved re-engineering phosphotriesterases by incorporating an artificial fluorinated amino acid and computational biology. This resulted in a thermo-stable protein with a longer half-life that retains all the detoxification capabilities of the original version.
The toxic nerve agent was demonstrated in the assassination this year of Kim Jong-nam, the half-brother of North Korea’s leader, a fraction of a drop of VX absorbed through the skin is fatal.
Even with expedient treatment, which involves administering a cocktail of atropine, oximes, and benzodiazepines, mortality rates are as high as 40 percent. V-series agents like VX, VR, VM, and Sarin — used in the Iran-Iraq War during the 1980s, in attacks in Japanese cities by the Aum Shinrikyo cult in the 1990s, and by the Syrian government on its citizens in 2013 — work by inhibiting the enzyme acetylcholinesterase (AChE), which catalyzes the breakdown of acetylcholine and other neurotransmitters.
Montclare’s current work, in collaboration with Dr. Tamara Otto at the U.S. Army Medical Research Institute of Chemical Defense (USAMRICD) at the Aberdeen Proving Ground, takes a two-pronged approach to modifying PTE: The researchers incorporated a protein/fluorine complex into PTE that confers stability and longer half-life; and, with Richard Bonneau, Director of the NYU Center for Data Science, they are using computational biology software suite Rosetta, which includes algorithms for modeling and analysis of protein structures, to re-engineer PTE’s active receptor site to “recognize” and bind more easily with a variety of V-agents.
Bonneau explains, “This work really represents an ideal application of computational protein design. We are really excited to work to make new design codes that mesh well with the bioengineering side of this project.”
Montclare said the team will use experimental results to investigate a second-generation set of fluorinated-PTE variants.
“This feedback-based approach will allow us to refine Rosetta to improve specific activity for the V-agents, making fluorinated-PTE variants even more effective based on insights from activity and stability data,” she said.






























