Neuroscientists Develop Novel Tool for Precise Gene Editing in Neurons
The CRISPR-Cas9 gene editing tool has had an extremely busy year. It recently removed genetic disorders from human embryos, targeted the “command center” of cancer, extracted HIV from a living organism, and pushed superbugs off the ledge. This is all thrilling news, but one of the most exciting potentials for CRISPR is the amazing advances it’s making possible in the field of medicine — particularly disease eradication.
More recently, this editing system has been used by neuroscientists at the Max Planck Florida Institute for Neuroscience (MPFI) for the first time ever, to precisely edit mature neurons.
This novel and powerful tool utilize the CRISPR-Cas9 system, a viral defense mechanism originally found in bacteria. When placed inside a cell such as a neuron, the CRISPR-Cas9 system acts to damage DNA in a specifically targeted place. The cell then subsequently repairs this damage using predominantly two opposing methods; one being non-homologous end joining (NHEJ), which tends to be error-prone, and homology-directed repair (HDR), which is very precise and capable of undergoing specified gene insertions. HDR is the more desired method, allowing researchers flexibility to add, modify, or delete genes depending on the intended purpose.
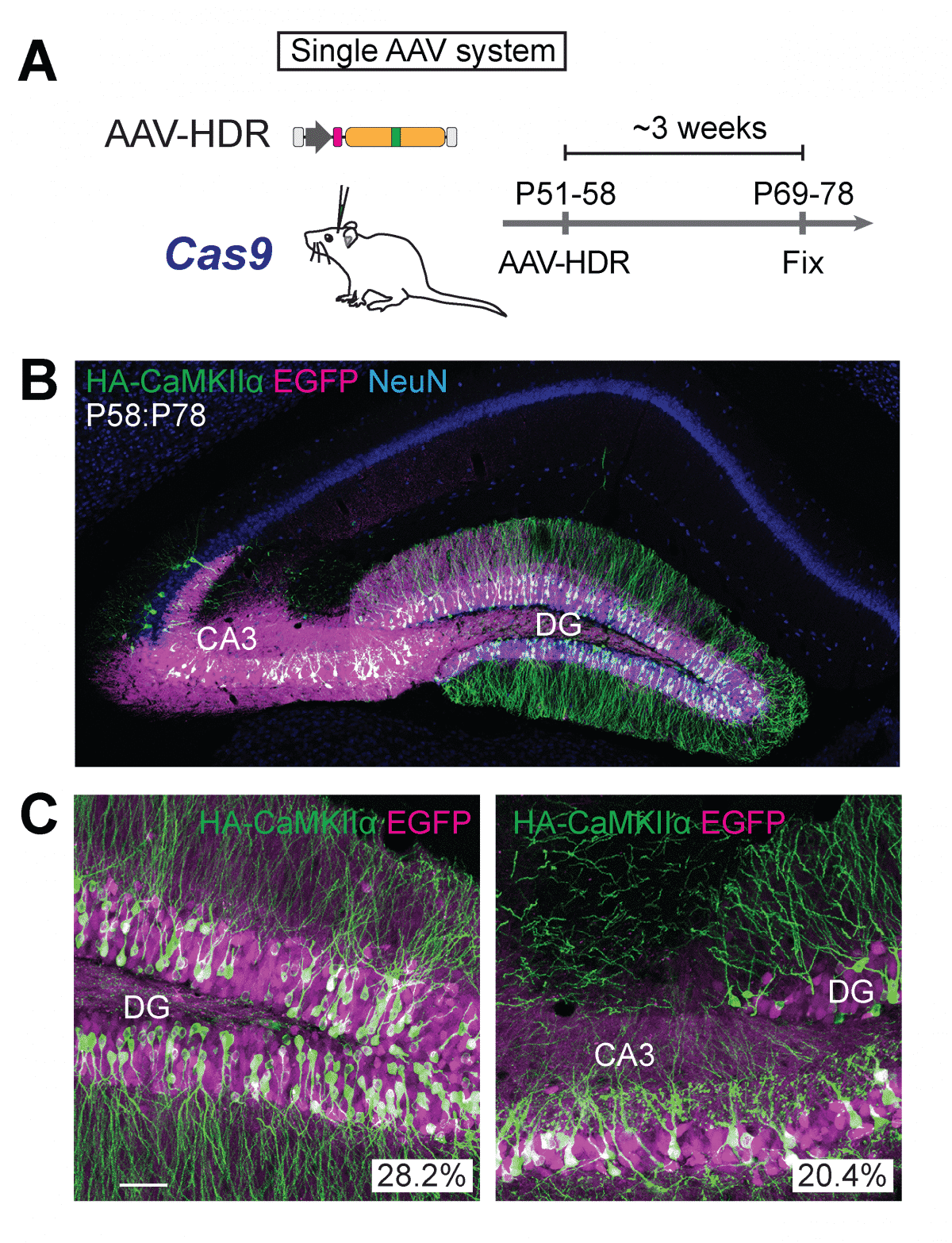
Until now, HDR has only been
feasible in cells that are dividing and proliferating in the body. To correct that shortcoming, the Max Planck team combined CRISPR-Cas9 with adeno-associated virus (AAV), a mild viral vector that’s often used for gene delivery. When they delivered the combo to mouse neurons, HDR occurred in an efficient manner, they reported. When they tested it in an aged mouse model of Alzheimer’s, it also worked.The brain is composed of distinct regions that differ in their functional roles and cellular architecture. For example, the hippocampus is an area well-known for its involvement in memory and its dysfunction in diseases such as Alzheimer’s, while the neocortex is involved in functions such as perception, consciousness, and language. The hippocampus has a single, curved cell layer, while the neocortex has six, stacked layers. At the cellular level, although they share canonical types of inhibitory interneurons (INs) and excitatory principal neurons (PNs), it remains largely unknown to what extent a single type in different brain regions displays similarity in gene expression, axonal shape, connectivity, and developmental origins.
Two postdoctoral researchers in Taniguchi’s lab, Yugo Ishino, Ph.D. and Michael Yetman, Ph.D., followed in his footsteps, painstakingly screening molecules until they found one that was reliably expressed in hippocampal chandelier cells – cadherin 6. Luckily, a mouse model already existed that allowed the team to take advantage of this gene expression and use it to compare the two cell populations.
Now with the ability to compare these two populations, the team found that chandelier cells in the hippocampus expand twice the size of axonal arbors in the neocortex and made twice as many connections as their counterparts. Additionally, the hippocampal chandelier cells were born several days earlier during embryonic development than the neocortical ones. Lastly, the team identified a gene, calretinin, in the hippocampal cells which were not expressed in the neocortical cells – suggesting the possibility that these cells exhibit different functional properties as well.
These results, Taniguchi explained, show that exquisite modifications of canonical neuronal types in different brain regions may contribute to their functional diversification. Future studies should elucidate the molecular and cellular mechanisms by which regional environment controls phenotypic variations of neuronal types.
In experimental models, the team used in utero electroporation, a technique that allowed them to insert the CRISPR/Cas9 system into prenatal brain cells that are still developing and dividing. Thus, the broken DNA is still being repaired via HDR, allowing the researchers to add a gene that made a protein of interest visible under the microscope. They were even able to reliably label two different proteins with distinct colors at the same time in the same cell. The researchers used a variety of imaging methods as well as DNA sequencing to confirm that the SLENDR method had truly and precisely knocked in the genes.
“I believe that SLENDR will be a standard tool for molecular and cellular neurobiology,” said Yasuda. “SLENDR provides a valuable means to determine the subcellular localization of proteins, and will help researchers to determine the function of the proteins.“