Oxford Spin-Out to Deploy Organs-on-Chip for Drug Safety Prediction
CN Bio Innovations, a spin-out from the University of Oxford, will be working with scientists from the FDA’s Centre for Drug Evaluation and Research in order to better predict drug safety in drug development by using human organs-on-chips.
Chief Executive Officer of CN Bio, Dr. Emma Sceats said: “This landmark collaborative deal outlines how we will work with FDA to build an evidence base detailing how Organs-on-Chips can improve the drug development process. Our aim is to create a step change in the speed and accuracy of tests which can predict the success of new drug candidates. We will work with FDA to compare Organs-on-Chips against current preclinical testing methods.”
FDA scientists will assess the use of the devices to provide precise data about how human cells and organs interact with new drugs, and their ability to accurately predict the success of drugs prior to human clinical trials.
The collaboration will characterize the performance of CN Bio’s Organs-on-Chips as a platform for potential use in drug development and regulatory evaluation, including safety testing prior to new drugs entering clinical trials.
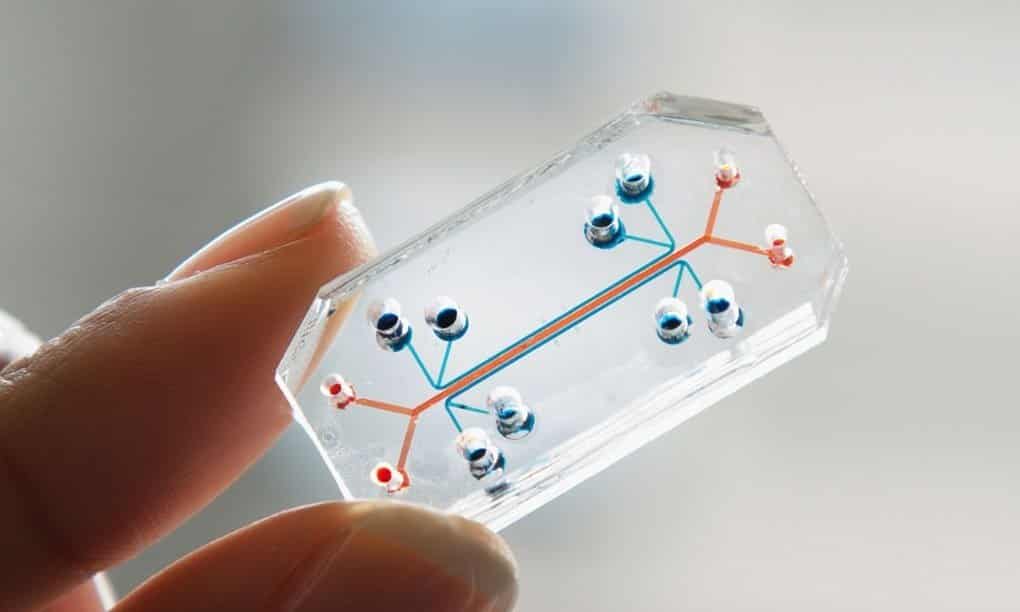
Organs-on-chips are miniature devices that recreate the natural physiological microenvironment of human cells within specific
tissues and organs. These devices can help study how drugs interact with specific human organs and have the potential to predict the activity and toxicity of drugs better than using animal models. Each organ-on-a-chip can then be linked to others to investigate systemic drug effects. The technology was pioneered at the MIT and Vanderbilt University, which have licensed it to CN Bio.This collaboration fits with FDA’s regulatory science efforts to advance the science of developing new tools, standards, and approaches to assess the safety, efficacy, quality, and performance of new therapeutics.
Although drug safety is carefully assessed and monitored throughout the drug development process, unexpected adverse events that raise concerns about overall product safety may be observed in late-stage clinical trials or the following approval. Liver toxicity is one of the most common causes of adverse side effects, preventing drugs from successfully completing clinical trials or leading to their withdrawal from the marketplace. FDA’s collaboration with CN Bio will focus on evaluating data, systems, and models to predict liver toxicity and drug safety.