CRISPR-Gold Found To Fix Duchenne Dystrophy Mutation In Mouse
The functions of CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) and CRISPR-associated (Cas) genes are essential in adaptive immunity in select bacteria and archaea, enabling the organisms to respond to and eliminate invading genetic material. These repeats were initially discovered in the 1980s in E. coli (9), but their function wasn’t confirmed until 2007 by Barrangou and colleagues, who demonstrated that S. thermophilus can acquire resistance against a bacteriophage by integrating a genome fragment of an infectious virus into its CRISPR locus.
Scientists have now developed a non-viral approach to delivering the CRISPR/Cas9 gene-editing system to cells since the conventional mode allows the Cas9 enzyme to persist in the cells, as there is no way to control Cas9 expression once the enzyme is in the cells. The Cas9 later will “chew up” parts of the genome, which can cause mutations.
Duchenne muscular dystrophy is a degenerative disease of the muscles caused by a lack of the protein dystrophin. In about a third of patients, the gene for dystrophin has small deletions or single base mutations that render it nonfunctional, which makes this gene an excellent candidate for gene editing. Researchers have previously used viral delivery of CRISPR-Cas9 components to delete the mutated exon and achieve clinical improvements in mouse models of the disease.
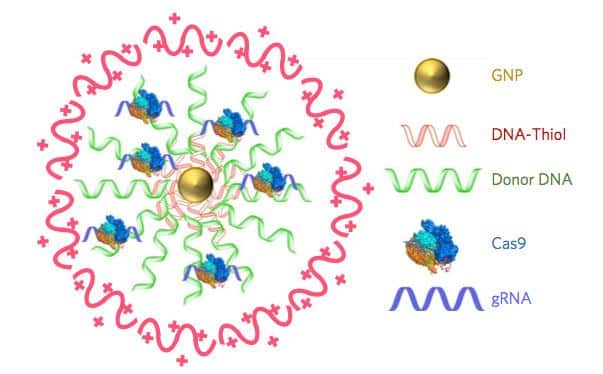
The platform, called CRISPR-Gold, uses gold nanoparticles to encapsulate all the elements needed for CRISPR/Cas9 gene editing and deliver them directly to cells. “CRISPR-Gold and, more broadly, CRISPR-nanoparticles open a new way for safer, accurately controlled delivery of gene-editing tools,” claims Irina Conboy, Ph.D., a bioengineering professor at the University of California, Berkeley (UC Berkeley), who co-led the CRISPR-Gold research alongside UC Berkeley’s professor Niren Murthy, Ph.D., and collaborators in the U.S. and Japan. “Ultimately, these techniques could be developed into a new medicine for Duchenne muscular dystrophy and a number of other genetic diseases.”
The new study shows that a single injection of CRISPR-Gold, as the new delivery system is called, into mice with Duchenne muscular dystrophy led to an 18-times-higher correction rate and a two-fold increase in a strength and agility test compared to control groups.
The research team created CRISPR-Gold by covering a central gold nanoparticle with DNA that they modified so it would stick to the particle. This gold-conjugated DNA bound the donor DNA needed for HDR, which the Cas9 protein and guide RNA bound to in turn. They coated the entire complex with a polymer that seems to trigger endocytosis and then facilitate escape of the Cas9 protein, guide RNA, and template DNA from endosomes within cells.
In the Nature Biomedical Engineering paper the UC Berkeley-led team describes use of the CRISPR-Gold system to deliver all the components required for CRISPR/Cas9-mediated HDR directly to a wide variety of therapeutically relevant cell types, including human embryonic stem cells, human induced pluripotent stem cells, and bone-marrow derived dendritic cells, as well as myoblasts from the mdx mouse model. Using the CRISPR-Gold platform, HDR efficiency was typically 3% to 4%, and there was minimal off-target DNA damage.
They then tested CRISPR-Gold HDR efficiency in vivo by injecting it into the muscles of a mouse model for Duchenne muscular dystrophy that does not produce dystrophin. A single dose of CRISPR-Gold restored dystrophin levels two weeks after injection and corrected the relevant point mutation to the wild-type sequence in about 5 percent of the copies of the gene for dystrophin in injected muscles. Injection of the Cas9 protein, guide RNA, and donor DNA without the particles corrected less than 1 percent of the copies of the gene for dystrophin. Muscle function also improved in mice treated with CRISPR-Gold.
Further analysis of the muscle tissues injected with CRISPR-Gold demonstrated robust dystrophin protein expression. And when compared with control animals, the CRISPR-Gold-treated mice showed a two-fold improvement in hanging time in a four-limb hanging test. Encouragingly, when the team carried out a deep sequencing analysis, they found that the degree of off-target DNA damage caused by CRISPR-Gold was no greater than the level of sequencing error (0.005–0.02%) found in a typical cell that hadn’t been exposed to CRISPR-Gold. “These results demonstrate that CRISPR-Gold can induce HDR in muscle tissue with minimal off-target genomic damage, effectively edit the dystrophin mutation in mdx mice to the wild-type sequence, and improve animals’ strength under clinically relevant conditions,” the researchers write.
They also tested for potential immunogenicity by measuring blood cytokine levels in treated animals, 24 hours and 2 weeks after CRISPR-Gold injection. The results showed no sign of inflammatory cytokine upregulation, and there was not weight loss, even after multiple injections, “suggesting that CRISPR–Gold can be used multiple times safely and that it has a high therapeutic window for gene editing in muscle tissue.” In fact, compared with virus-based CRISPR-Cas9 delivery, “nanoparticles are less immunogenic, less toxic and much cheaper to produce,” Dr. Murthy stated.
Professor Murthy, together with UC Berkeley co-authors Kunwoo Lee, Ph.D., and Hyo Min Park, Ph.D., have founded a company, GenEdit, to develop the CRISPR-Gold technology for human therapeutic applications and explore commercial applications of CRISPR-Gold. “GenEdit was formed about 16 months ago,” says Murthy. “GenEdit is heavily focused on new delivery vehicles for the Cas9 protein, and is developing platform technologies for this. The company has raised seed funding from well established venture capital companies. Partnerships with other companies are being considered.”
“Genetic diseases cause devastating levels of mortality and morbidity, and new strategies for treating them are greatly needed,” Murthy said. “CRISPR-Gold was able to correct disease-causing gene mutations in vivo, via the non-viral delivery of Cas9 protein, guide RNA and donor DNA, and therefore has the potential to develop into a therapeutic for treating genetic diseases.”
In parallel with the establishment of GenEdit, ongoing work at the UC Berkeley laboratories of Drs. Murthy and Conboy is focused on developing the next generation of particles that could deliver CRISPR-Gold into tissues from the bloodstream and preferentially target adult stem cells