The USFDA Approves First Finger-Prick Free Diabetes Device
Abbott has now gained the FDA clearance for its glucose monitoring device, which is cellphone-sized and that automatically takes care of checking a patient’s blood sugar, delivering lifesaving insulin as needed without requiring any finger-pricking.
The U.S. Food and Drug Administration said in a news release that it authorized Abbott’s FreeStyle Libre Flash Glucose Monitoring System for adults for sale in the U.S. The monitor is already is sold in 41 other countries and will likely launch stateside by the end of the year.
The Chicago-based company has not released the pricing information on the device, but it is expected to sell at a similar price point as those sold in Europe.
Abbott’s FreeStyle Libre Flash reduces the need for fingerstick testing, which is painful and inconvenient, by inserting a small sensor wire below the skin to continuously measure and monitor glucose levels. The device can be worn for up to 10 days.
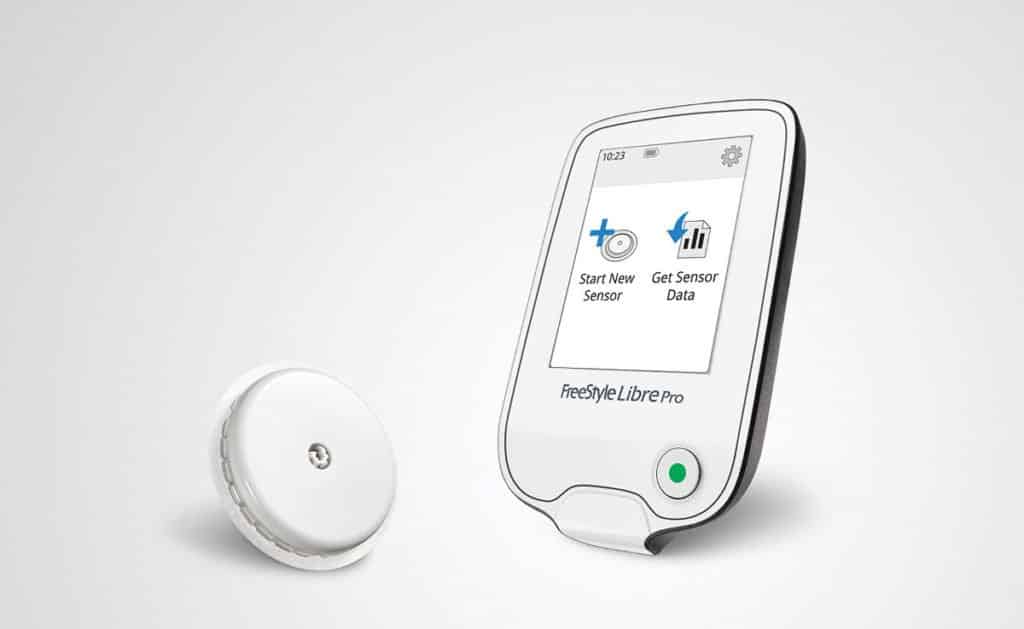
Most diabetes patients do not measure glucose as often as they should because of the discomfort caused by these kinds of tests, Jared Watkin, senior vice president of Abbott’s Diabetes Care unit said. According to studies, the majority
of people with diabetes test glucose levels less than three times a day. This device, however, is a long-lasting glucose sensor, which does not require fingerstick testing to ensure its accuracy.The FDA warned in a news release that patients could experience abnormally high or low glucose levels in cases where information provided by the device is inaccurate, and that information is used to make treatment decisions.
Abbott’s Kazemi, however, said Abbott is confident in the device’s accuracy. Patients using the devices check their glucose levels an average of 16 times a day in Europe, where the devices already are available. “It allows people to finally do what we’ve been asking them to do all along,” he said, referring to asking patients to regularly check their glucose levels.