JAM 2018
Joint Admission Test for M.Sc. 2018
Organizing Institute: IIT Bombay
Admission to M.Sc. (Two Year), Joint MSc-PhD., MSc-PhD Dual Degree
and other post-bachelor’s degree programmes
IITs and IISc are institutions of national importance and are well known, the world over, for quality education in engineering, science & technology and research in frontier areas. The aim of IITs and IISc is to build a sound foundation of knowledge, pursue excellence and enhance creativity in an intellectually stimulating environment. The vibrant academic ambience and well-equipped research infrastructure of IISc & IITs motivate the students to pursue Research and Development careers in frontier areas of basic sciences as well as interdisciplinary areas of science and technology.
Joint Admission Test for M.Sc. (JAM) is being conducted from 2004 to provide admissions to M.Sc. (Four Semesters), Joint M.Sc.-Ph.D., M.Sc.-Ph.D. Dual Degree, etc. Programmes at the IITs and Integrated Ph.D. Degree Programmes at IISc for consolidating Science as a career option for bright students. These postgraduate programmes at IITs and IISc offer high quality education in their respective disciplines, comparable to the best in the world. The curricula for these programmes are designed to provide opportunities to the students to develop academic talent leading to challenging and rewarding professional life.
ELIGIBILITY REQUIREMENTS (ERs) FOR ADMISSION
The candidates who qualify in JAM 2018 shall have to fulfill the following Eligibility Requirement (ER) for admissions in IITs.
- All candidates admitted through JAM should have a Bachelor’s degree.
- At least 55% aggregate marks, without rounding off, (taking into account all subjects, including Languages and Subsidiaries, all years combined) for General/OBC-NCL category Candidates and Foreign Nationals; at least 50% aggregate marks, without rounding off, (taking into account all subjects, including Languages and Subsidiaries, all years combined) for SC/ST and PwD Category Candidates in the qualifying degree.
- For Candidates with letter grades/CGPA (instead of percentage of marks), the equivalence in percentage of marks will be decided by the Admitting Institute(s).
- Proof of having passed the Qualifying Degree with the Minimum Educational Qualifications (MEQ) as specified by the admitting institute should be submitted by September 30, 2018.
- At the time of admission, all admitted candidates will have to submit a physical fitness certificate from a registered medical practitioner in the prescribed form. At the time of admission, the admitted candidates may also have to undergo a physical fitness test by a medical board constituted by the Admitting Institute. In case a candidate is not found physically fit to pursue his/her chosen course of study, his/her admission is liable to be cancelled.
Note:
(a) It will entirely be the responsibility of the Candidate to prove that he/she satisfies the Minimum Educational Qualifications (MEQs) and Eligibility Requirements (ERs) for Admissions.
(b) The Admitting Institute has the right to cancel, at any stage, the admission of a candidate who is found to have been admitted to a course to which he/she is not entitled, being unqualified or ineligible in accordance with the rules and regulations in force.
Table of Contents
Important Dates :
|
Commencement of ONLINE Registration and Application on JAM 2018 Website |
September 05, 2017 |
|
Last Date for Online Application Submission and Uploading of Documents on the Website |
October 10, 2017 |
|
Last Date for Payment of Application Fee through Online process |
October 10, 2017 |
|
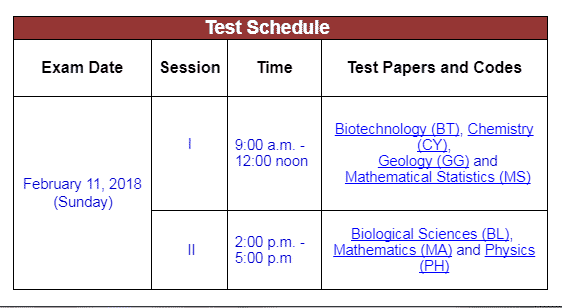
Date of JAM 2018 Examination |
February 11, 2018 |
|
Announcement of the Results of JAM 2018 |
March 20, 2018 |
General information
(a) JAM 2018 is open to all nationals (Indian/Foreign). Candidates seeking admission to the academic programmes covered under JAM 2018 need to appear in JAM 2018. There is no age restriction.
(b) JAM 2018 is a Computer Based ONLINE Examination. It will be held on 11 February 2018 (Sunday).
(c) For admission, foreign nationals are required to satisfy the rules and regulations of the admitting Institute(s) pertaining to foreign students. For further details, they are advised to contact the concerned Admitting Institute(s).
(d) To apply for admission to a desired programme, a candidate is required to qualify in the relevant Test Paper and also satisfy the Minimum Educational Qualifications (MEQs) and Eligibility Requirements (ERs) of the respective Academic Programme.
(e) The candidates who have either appeared or are due to appear in the final examination of their qualifying degree in 2018 are also eligible to appear in the test. By qualifying in JAM 2018, candidates can apply for provisional admission subject to the condition that: (a) all parts of their final examination shall be completed by the date of registration of the Admitting Institute, and (b) proof of having passed the qualifying degree with required eligibility, as specified by the Admitting Institute, will be submitted by 30 September 2018.
(f) Admission to most of the Academic Programmes at various institutes will be made on the basis of merit in JAM 2018.
(g) On the basis of performance in JAM 2018, for each test paper, separate merit lists will be prepared for General (GEN), OBC Non-Creamy Layer (OBC-NCL), SC, ST, and Persons with Disability (PwD) category candidates.
(h) Requests for the change of category, if any, with proper documentation, should reach the Organizing Institute latest by 10 May 2018. Requests received after this date will not be accepted under any circumstances.
(i) Candidates should note that mere appearance in JAM 2018 or being in the merit list of any test paper neither guarantees nor provides any automatic entitlement to admission. Qualified candidates will have to apply for admission as per the prescribed procedure. Admissions shall be made in order of merit in each category and depending on the number of seats available at the Admitting Institute(s).
(j) The list of academic programmes, number of seats, eligibility requirement and minimum educational qualifications of each of the programmesmentioned in this Information Brochure are subject to change, as per the policy of Admitting Institute(s).
(k) In this document, the phrases ‘Un-Reserved’ and ‘General’ are used interchangeably and they both mean the same.
(l) With regard to the interpretation of the provisions on any matter not covered in this Information Brochure, the decision of the Organizing Institute shall be final and binding on all the parties concerned.
(m) In all matters concerning JAM 2018, the decision of the Organizing Institute or the Organizing Chairman, JAM 2018 will be final and binding on all the applicants.
(n) Although JAM 2018 is held at different centres across country, Indian Institute of Technology Bombay is the Organizing Institute, and has the overall responsibility of conducting JAM 2018.In case of any claims or disputes arising in respect of JAM 2018, it is hereby made absolutely clear that the Mumbai High Court alone shall have the exclusive jurisdiction to entertain and settle any such disputes and claims.
EXAMINATION CITIES: JAM 2018
| Institute | Cities |
| IISc Bangalore | Bengaluru, Hubli, Hyderabad, Kannur, Kozhikode, Mangalore, Palakkad, Trissur |
| IIT Bombay | Ahmedabad, Goa, Mumbai, Nagpur, Nanded, Nasik, Pune, Vadodara |
| IIT Delhi | Faridabad, Ghaziabad, Greater Noida, Gurugram, Hisar, Indore, Jaipur, Jammu, Jodhpur, New Delhi |
| IIT Guwahati | Asansol-Durgapur, Dhanbad, Dibrugarh, Guwahati, Jorhat, Kalyani, Patna, Siliguri |
| IIT Kanpur | Agra, Allahabad, Bareilly, Bhopal, Kanpur, Lucknow, Varanasi |
| IIT Kharagpur | Bhubaneswar, Kharagpur, Kolkata, Raipur, Ranchi, Vijaywada, Visakhapatnam |
| IIT Madras | Chennai, Coimbatore, Ernakulam, Kollam, Kottayam, Madurai, Thiruvananthapuram, Tiruchirapalli, Tirunelveli, Warangal |
| IIT Roorkee | Dehradun, Jalandhar, Kurukshetra, NOIDA, Mohali, Moradabad, Roorkee |
ADMIT CARD
- a)Admit Card, bearing the Candidate’s Name, Registration Number, Photograph, Signature and Name(s) and Code(s) of the Test Paper(s) applied along with the Name and Address of the Test Centre allotted, will be available for download from JAM 2018 website from January 9, 2018 till Examination Date. Admit Cards will not be sent by post/E-mail.
- b)The Candidate should carefully examine the Admit Card for all the entries made therein. In case of any discrepancy, the Candidate should inform the Organizing Chairperson, JAM 2018, IIT Bombay immediately.
- c)If a candidate is not able to download the Admit Card, then the Chairman JAM of the respective IISc/IITs (See Appendix III of the JAM Brochure), under which the first choice Test City/Town of the candidate falls, may be contacted through Phone/Fax/E-mail, giving the Online Enrolment ID, Name, E-mail ID, Mobile Number, Mailing Address and City Code of the desired Test Centre (first choice) to get information about the Registration Number and the Name of the Test Centre allotted.
- d)A printout of the downloaded Admit Card must be brought to the test centre along with the original and valid photo identification. Please note that you have to give the details of this ID proof while filling the online application.
- e)No candidate will be permitted to appear in JAM 2018 examination without a valid Admit Card, and a valid and original ID.
- f)The Admit Card should be presented to the invigilators/JAM officials for verification.
- g)The Admit Card of JAM 2018 must be carefully preserved by the Candidate and produced at the time of admission, if required by the Admitting Institute.
- h)The Organizing Institute may withdraw the permission granted to a candidate to appear in JAM 2018, if it is found that the candidate is not eligible to appear in the exam, even though an Admit Card has been issued and is produced by the candidate before the Presiding Officer of the Test Centre.
Click Here to Apply Online
SYLLABUS
BIOLOGICAL SCIENCES (BL)
General Biology : Taxonomy of plants and animals; pro-and eukaryotic organisms; cell organelles and their function; multicellular organization; general physiology; energy transformations; internal transport systems of plants and animals; photosynthesis; respiration; regulation of body fluids and excretory mechanisms; reproductive biology; plant and animal hormones and their action; nervous systems; animal behavior; plant and animal diseases; Mendelian genetics and heredity; basics of developmental biology; biology of populations and communities; evolution; basic principles of ecology; genesis and diversity of organisms.
Basics of Biochemistry, Molecular Biology, Biophysics:
Buffers; trace elements in biological systems; enzymes and proteins; vitamins; biological oxidations, photosynthesis;carbohydrates and lipids and their metabolism; digestion and absorption; detoxifying mechanisms; nucleic acids;nucleic acid metabolism;nature of gene and its function; genetic code; synthesis of nucleic acids and proteins; regulation of gene expression; operons.
Structure of biomolecules; intra and intermolecular forces; thermodynamics and kinetics of biological systems; enzyme mechanisms and kinetics;principles of X-ray diffraction; IR- and UV- spectroscopy; analytical and biochemical techniques
Microbiology, Cell Biology and Immunology: Classification of microorganisms and their characterization; nutrient requirement for growth; laboratory techniques in microbiology; pathogenic microorganisms and disease; applied microbiology; viruses and fungi; microbial genetics;cell theory; cell architecture; cell division; types of chromosome structure; biochemical genetics- inborn errors of metabolisms; innate and adaptive immunity, antigen antibodies; principles of processes of development.
Mathematical Sciences: Mathematical functions (algebraic, exponential, trigonometric) and their derivatives (derivatives and integrals of simple functions); permutations and combinations; basic probability and volumetric calculations.
BIOTECHNOLOGY (BT)
The Biotechnology (BT) test paper comprises of Biology, Chemistry, Mathematics and Physics.
BIOLOGY (10+2+3 level)
General Biology: Taxonomy; Heredity; Genetic variation; Conservation; Principles of ecology; Evolution; Techniques in modern biology.
Biochemistry and Physiology: Carbohydrates; Proteins; Lipids; Nucleic acids; Enzymes; Vitamins; Hormones; Metabolism – Glycolysis, TCA cycle, Oxidative Phosphoryation; Photosynthesis. Nitrogen Fixation, Fertilization and Osmoregulation; Vertebrates-Nervous system; Endocrine system; Vascular system; Immune system; Digestive system and Reproductive System.
Basic Biotechnology: Tissue culture; Application of enzymes; Antigen-antibody interaction; Antibody production; Diagnostic aids.
Molecular Biology: DNA; RNA; Replication; Transcription; Translation; Proteins; Lipids and Membranes; Operon model; Gene transfer.
Cell Biology: Cell cycle; Cytoskeletal elements; Mitochondria; Endoplasmic reticulum; Chloroplast; Golgi apparatus; Signaling.
Microbiology: Isolation; Cultivation; Structural features of virus; Bacteria; Fungi; Protozoa; Pathogenic micro-organizms.
CHEMISTRY (10+2+3 level)
Atomic Structure: Bohr’s theory and Schrodinger wave equation; Periodicity in properties; Chemical bonding; Properties of s, p, d and f block elements; Complex formation; Coordination compounds; Chemical equilibria; Chemical thermodynamics (first and second law); Chemical kinetics (zero, first, second and third order reactions); Photochemistry; Electrochemistry; Acid-base concepts; Stereochemistry of carbon compounds; Inductive, electromeric, conjugative effects and resonance; Chemistry of Functional Groups: Hydrocarbons, alkyl halides, alcohols, aldehydes, ketones, carboxylic acids, amines and their derivatives; Aromatic hydrocarbons, halides, nitro and amino compounds, phenols, diazonium salts, carboxylic and sulphonic acids; Mechanism of organic reactions; Soaps and detergents; Synthetic polymers; Biomolecules – amino acids, proteins, nucleic acids, lipids and carbohydrates (polysaccharides); Instrumental techniques – chromatography (TLC, HPLC), electrophoresis, UV-Vis, IR and NMR spectroscopy, mass spectrometry.
MATHEMATICS (10+2 level)
Sets, Relations and Functions, Mathematical Induction, Logarithms, Complex numbers, Linear and Quadratic equations, Sequences and Series, Trigonometry, Cartesian System of Rectangular Coordinates, Straight lines and Family, Circles, Conic Sections, Permutations and Combinations, Binomial Theorem, Exponential and Logarithmic Series, Mathematical Logic, Statistics, Three Dimensional Geometry, Vectors, Matrices and Determinants, Boolean Algebra, Probability, Functions, limits and Continuity, Differentiation, Application of Derivatives, Definite and Indefinite Integrals, Differential Equations.
PHYSICS (10+2 level)
Physical World and Measurement, Elementary Statics and Dynamics, Kinematics, Laws of Motion, Work, Energy and Power, Electrostatics, Current electricity, Magnetic Effects of Current and Magnetism, Electromagnetic Induction and Alternating Current, Electromagnetic waves, Optics, Dual Nature of Matter and Radiations, Atomic Nucleus, Solids and Semiconductor Devices, Principles of Communication, Motion of System of Particles and Rigid Body, Gravitation, Mechanics of Solids and Fluids, Heat and Thermodynamics, Oscillations, Waves.
CHEMISTRY (CY)
PHYSICAL CHEMISTRY
Basic Mathematical Concepts: Functions; maxima and minima; integrals; ordinary differential equations; vectors and matrices; determinants; elementary statistics and probability theory.
Atomic and Molecular Structure: Fundamental particles; Bohr’s theory of hydrogen-like atom; wave-particle duality; uncertainty principle; Schrodinger’s wave equation; quantum numbers; shapes of orbitals; Hund’s rule and Pauli’s exclusion principle; electronic configuration of simple homonuclear diatomic molecules.
Theory of Gases: Equation of state for ideal and non-ideal (van der Waals) gases; Kinetic theory of gases; Maxwell-Boltzmann distribution law; equipartition of energy.
Solid state: Crystals and crystal systems; X-rays; NaCl and KCl structures; close packing; atomic and ionic radii; radius ratio rules; lattice energy; Born-Haber cycle; isomorphism; heat capacity of solids.
Chemical Thermodynamics: Reversible and irreversible processes; first law and its application to ideal and nonideal gases; thermochemistry; second law; entropy and free energy; criteria for spontaneity.
Chemical and Phase Equilibria: Law of mass action; Kp, Kc, Kx and Kn; effect of temperature on K; ionic equilibria in solutions; pH and buffer solutions; hydrolysis; solubility product; phase equilibria-phase rule and its application to one-component and two-component systems; colligative properties.
Electrochemistry: Conductance and its applications; transport number; galvanic cells; EMF and free energy; concentration cells with and without transport; polarography; concentration cells with and without transport; Debey-Huckel-Onsagar theory of strong electrolytes.
Chemical Kinetics: Reactions of various order; Arrhenius equation; collision theory; transition state theory; chain reactions – normal and branched; enzyme kinetics; photochemical processes; catalysis.
Adsorption: Gibbs adsorption equation; adsorption isotherm; types of adsorption; surface area of adsorbents; surface films on liquids.
Spectroscopy: Beer-Lambert law; fundamental concepts of rotational, vibrational, electronic and magnetic resonance spectroscopy.
ORGANIC CHEMISTRY
Basic Concepts in Organic Chemistry and Stereochemistry: Electronic effects (resonance, inductive, hyperconjugation) and steric effects and its applications (acid/base property); optical isomerism in compounds with and without any stereocenters (allenes, biphenyls); conformation of acyclic systems (substituted ethane/n-propane/n-butane) and cyclic systems (mono- and di-substituted cyclohexanes).
Organic Reaction Mechanism and Synthetic Applications: Chemistry of reactive intermediates (carbocations, carbanions, free radicals, carbenes, nitrenes, benzynes etc …); Hofmann-Curtius-Lossen rearrangement, Wolff rearrangement, Simmons-Smith reaction, Reimer-Tiemann reaction, Michael reaction, Darzens reaction, Wittig reaction and McMurry reaction; Pinacol-pinacolone, Favorskii, benzilic acid rearrangement, dienone-phenol rearrangement, Baeyer-Villeger reaction; oxidation and reduction reactions in organic chemistry; organometallic reagents in organic synthesis (Grignard, organolithium and organocopper); Diels-Alder, electrocyclic and sigmatropic reactions; functional group inter-conversions and structural problems using chemical reactions.
Qualitative Organic Analysis: Identification of functional groups by chemical tests; elementary UV, IR and 1H NMR spectroscopic techniques as tools for structural elucidation.
Natural Products Chemistry: Chemistry of alkaloids, steroids, terpenes, carbohydrates, amino acids, peptides and nucleic acids.
Aromatic and Heterocyclic Chemistry: Monocyclic, bicyclic and tricyclic aromatic hydrocarbons, and monocyclic compounds with one hetero atom: synthesis, reactivity and properties.
INORGANIC CHEMISTRY
Periodic Table: Periodic classification of elements and periodicity in properties; general methods of isolation and purification of elements.
Chemical Bonding and Shapes of Compounds: Types of bonding; VSEPR theory and shapes of molecules;
hybridization; dipole moment; ionic solids; structure of NaCl, CsCl, diamond and graphite; lattice energy.
Main Group Elements (s and p blocks): General concepts on group relationships and gradation in properties; structure of electron deficient compounds involving main group elements.
Transition Metals (d block): Characteristics of 3d elements; oxide, hydroxide and salts of first row metals; coordination complexes: structure, isomerism, reaction mechanism and electronic spectra; VB, MO and Crystal Field theoretical approaches for structure, color and magnetic properties of metal complexes; organometallic compounds having ligands with back bonding capabilities such as metal carbonyls, carbenes, nitrosyls and metallocenes; homogenous catalysis.
Bioinorganic Chemistry: Essentials and trace elements of life; basic reactions in the biological systems and the role of metal ions, especially Fe2+, Fe3+, Cu2+ and Zn2+; structure and function of hemoglobin and myoglobin and carbonic anhydrase.
Instrumental Methods of Analysis: Basic principles; instrumentations and simple applications of conductometry, potentiometry and UV-vis spectrophotometry; analysis of water, air and soil samples.
Analytical Chemistry: Principles of qualitative and quantitative analysis; acid-base, oxidation-reduction and complexometric titrations using EDTA; precipitation reactions; use of indicators; use of organic reagents in inorganic analysis; radioactivity; nuclear reactions; applications of isotopes.