Stimwave Technologies Incorporated, a medical device manufacturer and independent research institute headquartered in Miami Beach, Fla., has received FDA clearance for its microtechnology neurostimulator device. Now, patients implanted with the device may undergo full-body MRI scans without needing to remove the implant.
Neurostimulation is an alternative treatment to opioids and invasive surgery for patients with chronic pain. MRI-compatibility makes such devices an option for patients with chronic pain who were previously ineligible because they might need diagnostic imaging in the future.
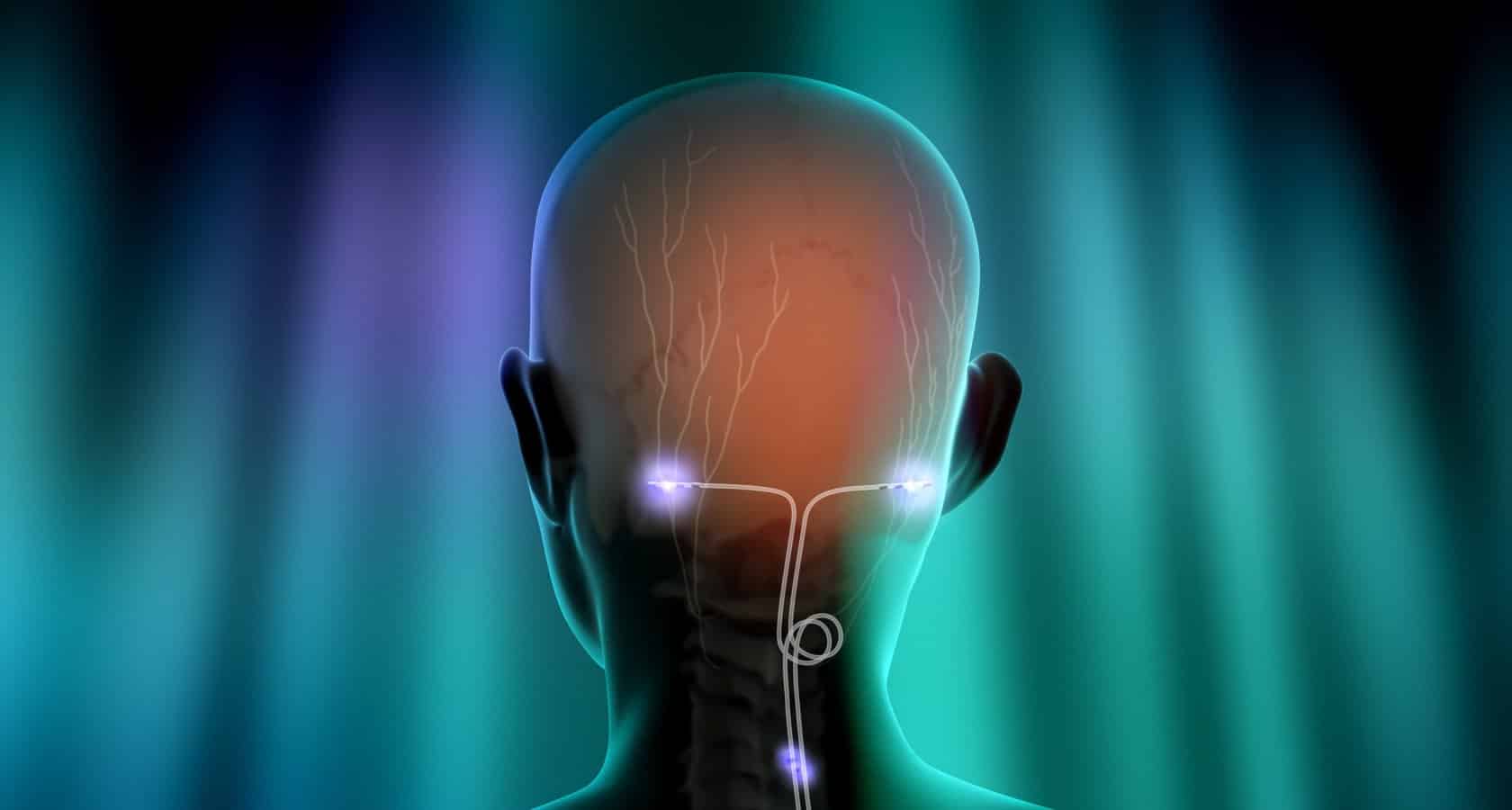
Stimwave’s wireless device- StimQ Peripheral Nerve Stimulator system, is designed to treat chronic pain, including shoulder pain, mid- and lower back pain and neuropathy in the upper and lower extremities. It delivers small electrical pulses through a set of electrodes to the targeted nerve. It is powered by a small external unit.
The device is placed through a needle and is designed to stimulate any peripheral nerve below the head and outside the spinal cord.
“This is great news for many chronic pain patients who previously did not have a minimally-invasive implant option available for peripheral nerve-related pain and will continue to require frequent MRI scans throughout their body for management of their pain and monitoring of their current and
future medical needs,” said Dr. Konstantin Slavin, a professor of neurosurgery at University of Illinois at Chicago, in the statement.