Solid tumours do not always have to be cancer tumours. But in cases that they are, they could be hugely difficult to deal with, irrespective of whether they are benign or malignant. They are abnormal masses of tissues that generally do not contain cysts or liquid areas. These tumours are formed in the case of many forms of cancers such as mesothelioma, ovarian cancer etc.
Presently a UK based company, Cell Medica is in the process of trying to enhance the existing T cell therapies for the treatment of solid tumors.
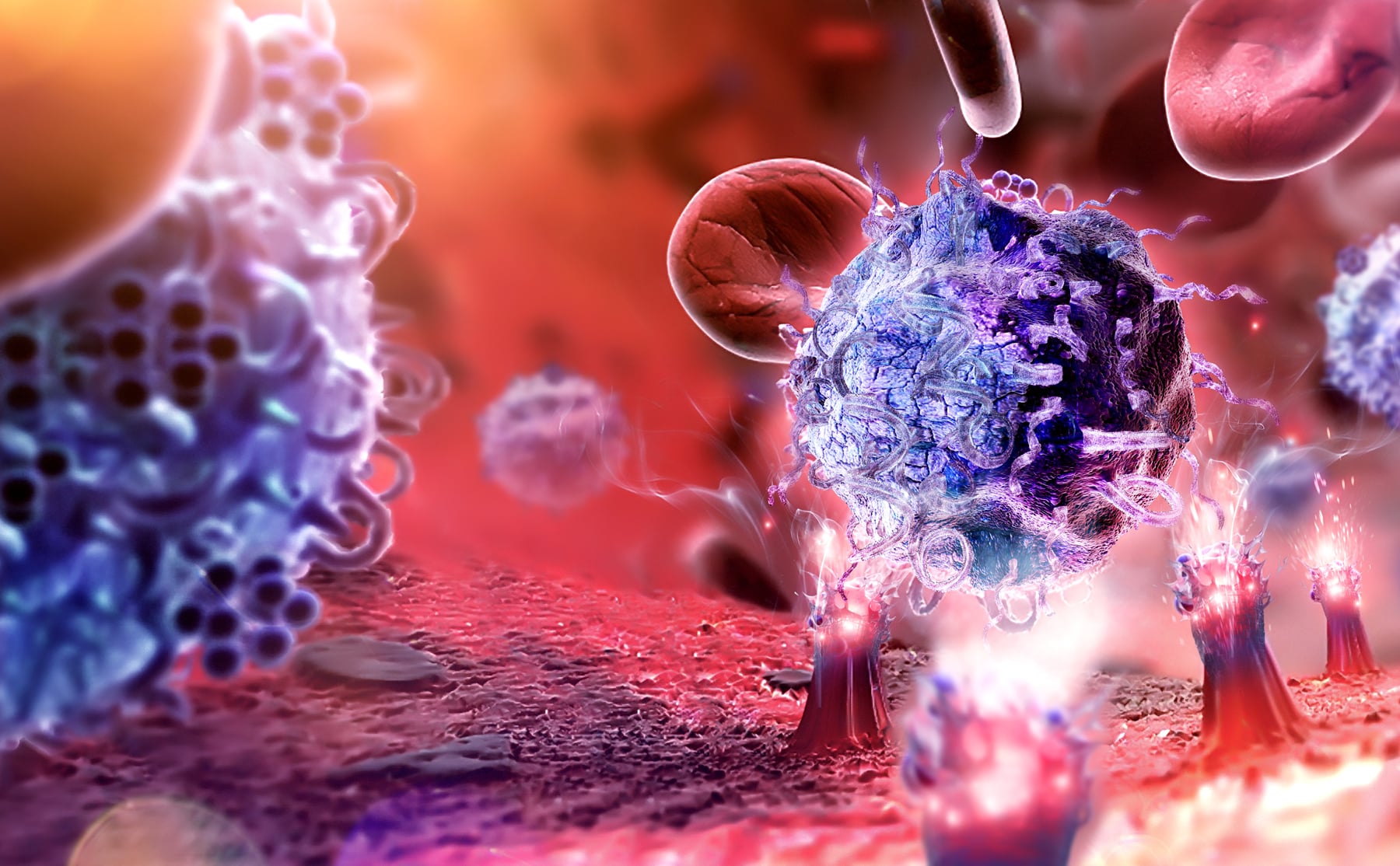
T cells are practically living drugs and the therapy is a living therapy. The T cell therapies have in the past proven to be highly effective in the treatment of cancer tumors. The therapies involve engineering of the patients’ T cell and reintroducing them into their body where they are allowed to multiply, seek out specific cancer cells or tumours and ultimately destroy them.
The company seems all set to advance in this direction as it just acquired Catapult Therapy TCR, a subsidiary of Cell and Gene Therapy (CGT) Catapult for its WT1-TCR therapy. The company already possesses the Dominant T cell receptor (TCR) platform technology, which it licensed from University College London (UCL
) last year. The WT1-TCR therapy that resulted from a research at UCL and the Imperial College London was spun out into a company by CGT Catapult. The technology is designed to engineer the patient’s own T cells to target the WT1-antigen, which is expressed in a number of cancers.Cell Medica plans to retool the technology using its Dominant TCR platform to treat solid tumors such as mesothelioma and ovarian cancer. It expects to start clinical trials with the upgraded WT1-TCR version to treat solid tumors in late 2018.
“Our objective is to show how we can enhance any existing TCR cell therapy with the Dominant TCR technology to create a more effective treatment for patients with solid tumors who otherwise have a very poor prognosis,” read a statement issued by the CEO of Cell Medica, Gregg Sando.
Cell Medica is also setting up its cell therapy manufacturing at CGT
Catapult’s GMP manufacturing facility where both the companies are expected to come together and develop a commercial scale production process for the engineered technology.