Lead Associate Jobs at Genpact – Regulatory Affairs | Life sciences jobs | Apply Now
Ready to grow your career with industry-leading Lead Associate jobs? Genpact is offering impactful Life sciences jobs through its global Genpact careers platform. This role places you at the heart of regulatory affairs operations, supporting global pharmaceutical submissions and compliance excellence.
- Job Title: Lead Associate
- Location: India-Mumbai
About the Company:
Genpact is a global professional services company delivering advanced digital, analytics, and operational solutions. Known for innovation and scale, Genpact careers offer rewarding Life sciences jobs that combine regulatory expertise with future-ready technologies.
Job Details:
This Lead Associate role focuses on regulatory affairs operations for the US market. Professionals in these Lead Associate jobs will manage regulatory publishing activities, ensure compliance with industry standards, and support submission processes while contributing to Genpact’s life sciences service excellence.
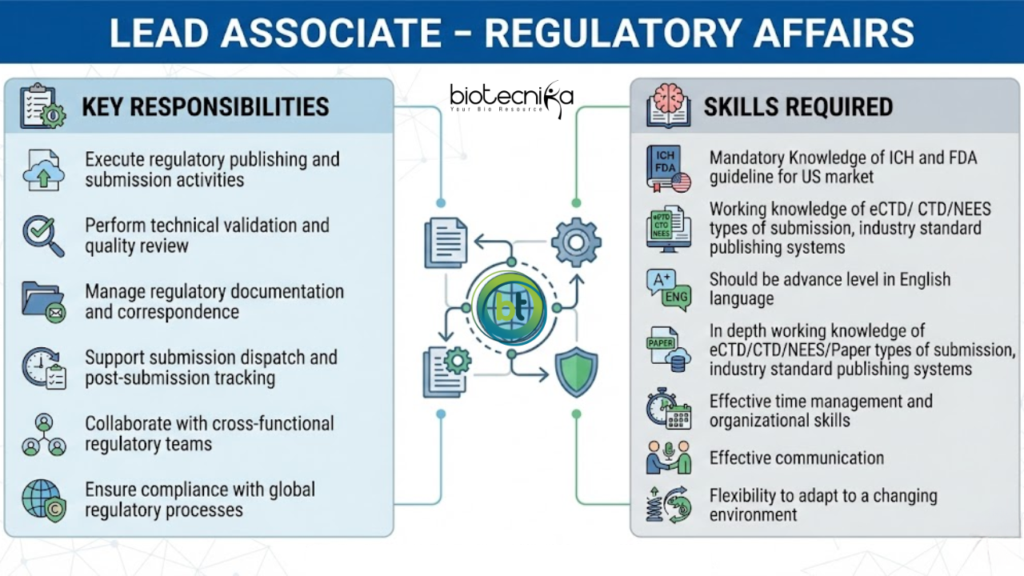
Key Responsibilities:
- The Role demands for a publisher with demonstrated ability to execute responsibility in a highly regulated & process driven environment, the Person will be responsible for all the activities related to.
- Publishing and performing technical validation of eCTD for US submissions.
- Performing final technical quality review.
- Dispatching submission to the relevant authority (eCTD/CTD/NeeS/Paper) or affiliate so that affiliate can dispatch to authority.
- Performing post-submission processing activities such as receiving acknowledgement from authority of submission receipt; capturing and the electronic receipt and metadata in RIM; communicating submission receipt to key stakeholders.
- Capturing submissions-related correspondence from health authorities, such as uploading documentation, commitments and metadata.
Educational Requirements:
- Bachelor’s degree, preferably or related Life science discipline required with relevant experience in the pharmaceutical industry.
Skills Required:
- Mandatory Knowledge of ICH and FDA guideline for US market.
- Working knowledge of eCTD/ CTD/NEES types of submission, industry standard publishing systems.
- Should be advance level in English language.
- In depth working knowledge of eCTD/CTD/NEES/Paper types of submission, industry standard publishing systems.
- Effective time management and organizational skills.
- Effective communication.
- Flexibility to adapt to a changing environment.
Genpact offers a strong career pathway for professionals seeking growth-driven Lead Associate jobs. With global exposure, innovation-led culture, and meaningful Life sciences jobs, Genpact careers provide an excellent platform to advance in regulatory affairs.































