Biosimilars and Biologic Drug Evaluation: Regulatory Guidelines, Clinical Research, and Safety Assessment
They look similar, cost less, and save lives, but what truly proves a Biosimilar is as safe and effective as Biologic Drugs?
Hello, there.
Imagine a world where life-saving Medications could be made more affordable to the common public, without compromising on the effectiveness or safety of the drugs. Continuing, imagine a world where patients who have long battled Diabetes, AIDs (Autoimmune Disorders), any severe Chronic disease, or Cancer no longer have to face the weight of expensive treatment expenditures. Many of these challenges today are closely tied to the high cost and complexity of Biologic Drugs.
Shocked? Well, this is the promise of Biosimilars in today’s technologically evolving Life Sciences world. At this point, a fundamental question arises about what Biosimilars are, and how they differ from traditional medicines.
Biologics have already been transforming modern medicine by providing targeted and precise treatments for such complicated diseases & disorders. But there’s a loophole: these Medications and treatments are very expensive, which often limits access for numerous patients worldwide.
Hence, “Biosimilars,” which are highly similar versions of approved Biologic drugs, are revolutionizing the healthcare landscape by offering more affordable, safer, and more effective alternatives to Biologic drugs. This growing discussion around Biologics vs Biosimilars is reshaping how modern therapies are developed, regulated, and accessed.
Developing these Medications and Therapies is not a cake walk; it requires strict Regulatory frameworks, comprehensive Clinical Trials, and rigorous Scientific Research. At the core of this process lies extensive Clinical Research, which validates safety, efficacy, and similarity. This, in turn, is to be maintained to ensure the drug’s quality, safety, and efficacy.
In our country, this field of Biologic Drugs is gaining momentum rapidly. The local Pharmaceutical companies are striving to meet the domestic demand for Biosimilar and Biologic Drugs and are emerging as global Pharmaceutical leaders in Biosimilar innovation and development.
With the possibilities of Biosimilar Drugs becoming clear, a question might arise. How can these complicated and specialized Drugs match the effectiveness and safety of the original Biologic Drugs?
Well, the simple answer lies in the careful scientific evaluation they undergo, their unique structure, and the strict Regulatory Authorities that guide their approval before being made available to the public.
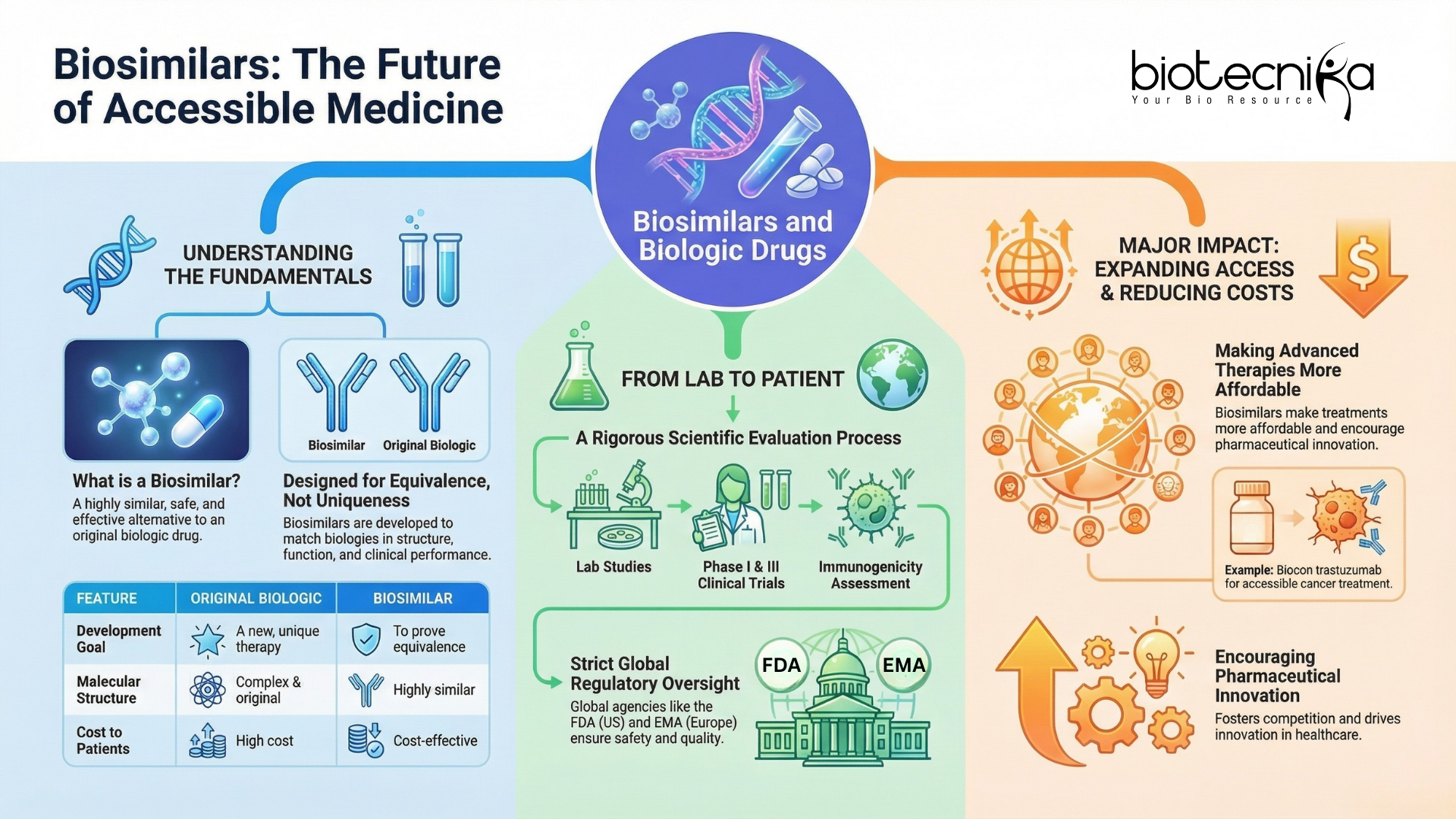
To briefly understand the world of Biosimilars, you need to explore what sets them apart from traditional drugs and the rigorous processes behind their development.
Understanding Biosimilars & Biologic Drugs
Unlike the conventional Generic Drugs, which are simple Chemical duplicates of original drugs, Biologic drugs are large and complex compounds produced in living cells or organisms. This complexity makes the exact replication of Biologic drugs impossible.
To clearly answer what Biosimilars are, they are Biologic drugs designed to closely match an already approved Biologic in structure, clinical, as well as functional performance.
Instead, a Biosimilar drug should exhibit high similarity in Clinical performance, structure, and function, with no or minimal clinical differences from the reference Biologic drugs.
The evaluation process of Biosimilars involves the following:
- Immunogenicity assessment: Evaluating potential immune responses to the drugs.
- Functional studies: To ensure Biological activity matches the original Biologic drugs.
- Physicochemical characterization: Confirming molecular modifications as well as the structure of Biosimilar.
These essential steps ensure Biosimilars provide effective, safe, and predictable therapy for patients and are more affordable than Biologic Drugs.
Understanding what Biosimilars are also requires comparing how they are evaluated against their reference Biologic Drugs. To understand why Biosimilars are becoming trusted therapeutics worldwide, here’s a table comparing their evaluation with that of their Biologic drugs.
Biosimilars vs Biologic Drugs: Key Evaluation Parameters
The comparison below highlights the key differences in the ongoing debate of Biologics vs Biosimilars.
|
Evaluation Aspect |
Reference Biologics |
Biosimilars |
|
Development Goal |
Establish original safety, efficacy, and quality |
Demonstrate high similarity to an approved biologic |
|
Molecular Structure |
Unique, complex structure |
Highly similar but not identical |
|
Analytical Testing |
Extensive characterization during initial development |
Highly detailed comparative analysis with the reference product |
|
Preclinical Studies |
Required to establish biological activity and safety |
Conducted mainly to confirm similarity |
|
Clinical Trials |
Full Phase I–III trials for all indications |
Comparative PK/PD and confirmatory Phase III trials |
| Indication Approval | Each indications are tested separately | Indications may be extrapolated with scientific justification |
|
Immunogenicity Assessment |
Evaluated during clinical development |
A critical focus during development and post-approval |
|
Regulatory Focus |
Benefit–risk evaluation of a new molecule |
Proof of similarity with no meaningful clinical differences |
|
Cost to Patients |
High due to innovation and R&D costs |
Lower due to reduced development time and costs |
This comparison shows that Biosimilars follow a distinct yet equally rigorous pathway, with a strong emphasis on similarity, safety, and clinical performance rather than on shortcut development.
Regulatory Guidelines for Biosimilars and Biologic Drugs: Global and Indian Perspectives
Regulatory Authorities maintain strict guidelines to evaluate Biosimilars and to protect patient safety worldwide. Globally, Biosimilars are regulated through stepwise and well-defined Regulatory frameworks established by Domestic or International Regulatory Authorities. These guidelines focus on analytical comparability, targeted clinical trials, and long-term safety monitoring.
Regulatory frameworks play a crucial role in defining the balance between innovation and accessibility in Biologics vs Biosimilars. A key Regulatory concept is the extrapolation of indications, which allows approval for multiple uses once Scientific similarity is justified.
Global Guidelines
Regulatory Authorities like EMA (European Medicines Agency) in Europe and FDA (Food and Drug Administration) in the United States follow a stepwise approach for approval of any Pharmaceutical product:
- Clinical Trials: Comparative studies on Pharmacodynamics, Pharmacokinetics, safety, and efficacy.
- Extrapolation of Indications: Approval for multiple indications based on data obtained from primary Clinical studies of the drugs.
- PreClinical & Analytical Assessment: Comprehensive characterization of the molecular function and structure of the drugs.
Indian Regulations
India is proudly becoming a center for Biosimilar R&D (Research & Development). The major Regulatory Authority, CDSCO (Central Drugs Standard Control Organization), along with the DBT (Department of Biotechnology), has established a proper Regulatory structure for the approval of Biosimilars in India.
Our country’s approach emphasizes mainly:
- Promotion of domestic drug manufacturing to improve patient access and reduce expenditure.
- Preclinical and Clinical Trials comparability with the reference drug product.
- Robust PV (Pharmacovigilance) to monitor long-term safety of the patients.
Indian leading Pharmaceutical companies, including Intas, Biocon, Zydus Cadila, as well as Dr. Reddy’s Laboratories, have developed Biosimilars for important therapies such as Cancer drugs, insulin, & mAb (Monoclonal Antibodies).
India is projected to have the third-largest Biosimilar market globally, supplying both domestic and international drug demand.
Clinical Research and Safety Assessment of Biologic Drugs and Biosimilars
Clinical Research for Biosimilars is designed to validate and confirm therapeutic safety and equivalence, rather than rediscover drug efficacy from scratch. Focused Clinical Research ensures that Biosimilars deliver the same therapeutic outcomes as their Biologic Drugs.
- Immunogenicity Monitoring: Detects any potential immune reactions induced by the drug to ensure patient safety.
- Clinical Trials (Phase I): Compare Pharmacodynamics and Pharmacokinetics with the reference Biologic drugs.
- Clinical Trials (Phase III): Confirm safety and efficacy in patients across relevant indications.
Even after approval of the drugs, Post-Marketing Surveillance is crucial to track any long-term or rare adverse effects caused by the drugs.
In India, the rise of advanced Biotechnology facilities and Clinical Research infrastructure has strengthened the ability to conduct high-quality trials aligned with internationally accepted standards.
Impact of Biosimilars and Biologic Drugs on Healthcare in India
Biosimilars are reshaping the Healthcare sector by reducing treatment expenditures, encouraging innovation & competition, and expanding patient access.
In India, the impact of biosimilars is significant. Affordable Biosimilars have enhanced access to life-saving treatments for chronic diseases & cancer, thereby reducing the financial burden on healthcare systems and patients.
Our country’s strength in cost-efficient drug manufacturing, combined with strict Regulatory oversight, has positioned India as both a global supplier and a major consumer of Biosimilars. This has strengthened India’s contribution to global health equity.
Global and Indian Impact
Biosimilars are transforming Healthcare globally by:
- Expanding Accessibility: Biosimilars provide life-saving treatments and medications in areas or countries with limited Healthcare resources and finances.
- Reducing Treatment Expenses: Cost-effective drug alternatives increase accessibility to essential therapeutics for the general public.
- Encouraging Innovation & Development: Leading Pharmaceutical Companies invest in futuristic Analytical Technology, advanced manufacturing, and process optimization.
In India, the impact of Biosimilars is quite significant. For example, Biocon developed a Biosimilar of the Biologic Drug ‘trastuzumab.’ This drug is utilized for the treatment of HER2-positive breast and gastric cancers. This Biosimilar has drastically reduced treatment expenditure, thereby making the drug accessible to thousands of patients who previously could not afford the expensive Biologic Drugs.
By providing affordable and effective drug alternatives, Indian Biosimilar manufacturers contribute to equitable Healthcare access and enhance India’s position in the global Biotechnology & Pharmaceutical market.
Challenges and Future Outlook of Biologic Drugs and Biosimilars
Advancements in Clinical Research are also driving the development of Next-Gen Biosimilars, improving patient convenience globally. Despite tremendous progress, certain challenges remain, such as:
- Next-Generation Biosimilars: Improved dosing convenience, delivery methods, and stability are areas of active Research.
- Regulatory Harmonization: Differences in global Regulatory approval processes can delay international adoption of the Biosimilar.
- Patient & Physician Awareness: Education is essential to building trust in Biosimilars worldwide.
Looking ahead, India is poised to expand its role in global Biosimilar production. Emerging Technologies, including AI (Artificial Intelligence) and ML (Machine Learning), are being applied to optimize drug R&D and drug production. It also aids in streamlining Regulatory submissions and in predicting immunogenicity, promising even safer and more efficient Biosimilars.
Biosimilars represent a remarkable convergence of Scientific innovation, Regulatory oversight, and patient-centered care. Their careful evaluation, from laboratory analysis and characterization to Clinical Trials and post-marketing monitoring, ensures safety, efficacy, and accessibility.
Biosimilars are enabling more patients worldwide to access life-saving therapeutics at lower costs. With advancing Technology, strong Regulations, and growing Clinical expertise, the future of Biosimilars is bright and promising. These therapeutics are not just cost-effective; they are a symbol of equitable Healthcare, hope for millions of patients, and Scientific progress.
As Science, Regulation, and innovation continue to evolve, Biosimilars will play an increasingly vital role in shaping a future where life-saving Medicines are safe, effective, and available to all.
Key Takeaways
- Biosimilars are highly similar versions of Biologic drugs, offering affordable and safer drug alternatives.
- Rigorous evaluation includes molecular analysis, Clinical Trials, and ongoing safety monitoring.
- India is a growing hub for Biosimilar Research, manufacturing, and global export.
- Biosimilars improve Healthcare access, reduce costs, and foster innovation.
- Challenges include awareness, Regulatory alignment, and next-generation development, but technological advances provide solutions.




























