Regulatory Affairs Jobs | Astellas is Hiring in Bangalore | Apply Now
Looking to advance your Biology Career in the global pharmaceutical industry? Astellas is hiring a Regulatory Affairs Specialist – CMC Small Molecules at its Global Capability Center in Bengaluru. This Regulatory Affairs Job is ideal for life science professionals seeking microbiology jobs, hands-on exposure to global CMC submissions, regulatory compliance, and product lifecycle management in a multinational pharma environment.
Job Details
- Job Title: Regulatory Affairs Specialist – CMC Small Molecules
- Location: Bengaluru, IND
- Job Type: Full-time
About the Company
Astellas Pharma Inc. is a global pharmaceutical company dedicated to turning innovative science into value for patients. Through its Global Capability Centers (GCCs) in India, Poland, and Mexico, Astellas strengthens regulatory, scientific, and operational excellence. The company focuses on oncology, urology, immunology, and rare diseases. Astellas emphasizes compliance, innovation, and patient-centric drug development. Its Bengaluru GCC plays a key role in global regulatory and CMC activities. Astellas offers strong growth opportunities for professionals in Regulatory Affairs Jobs promoting an impactful Biology career.
Key Responsibilities
- Contribute to preparation, compilation and transmittal of CMC submissions according to the defined schedules and meet both Health Authority and Astellas established SOPs / guidance.
- Contribute to the regulatory impact assessment of proposed CMC changes and monitors the progress of the CMC changes with regulatory impact.
- Ensure proper CMC document, including version control and metadata, and dossier management.
- Identify, communicate and escalate potential issues to CMC RA Scientific Lead.
- Act as CMC RA Deputy to support CMC RA Scientific Lead for both development programs and commercial products for regulator filing.
- Contribute to collecting, interpretation, archiving, and knowledge sharing of regulatory requirements for both BLA/MAA and post-approval submissions.
- Conduct all activities with an unwavering focus on compliance, including staying current on all training.
- Carry out all assignments to the standards of efficiency, innovation, accuracy and safety in accordance with company and regulatory requirements.
- Additional activities may be assigned by the supervisor.
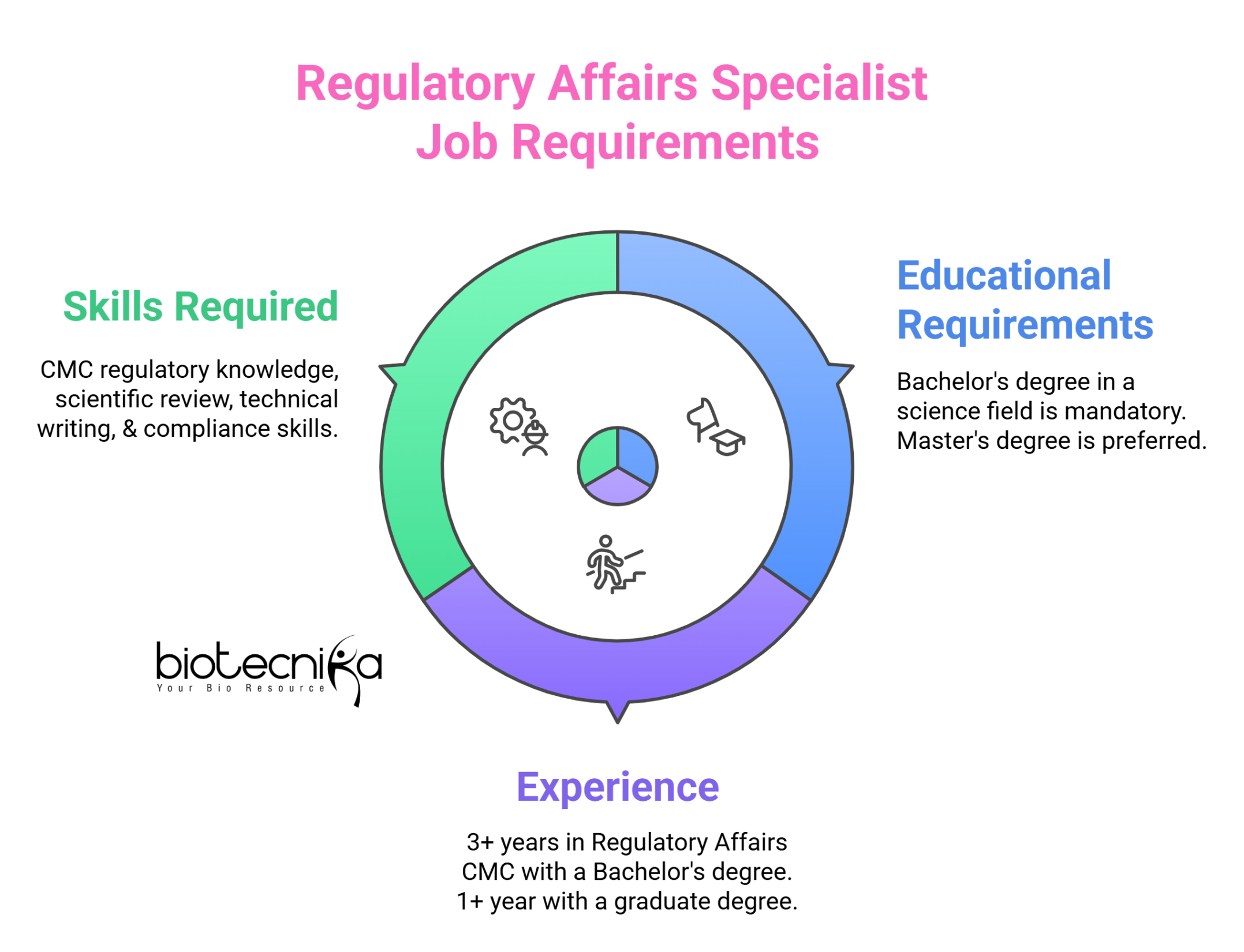
Required Qualifications
- Bachelor’s (minimum) or graduate degree (preferred) in biology, engineering, or a related field. Fields of study include Biology, Microbiology, or Biochemistry or other similar scientific discipline.
- At least three (3) years of relevant experience in regulatory CMC, or biological or vaccine research, manufacturing, testing; or related fields for candidates with a bachelor’s degree or 1 year with a graduate degree.
Skills Required
- The ideal candidate must have the ability to critically review detailed scientific information and assess whether technical arguments are presented clearly, and conclusions are adequately supported by data.
- The ideal candidate must have strong organizational skills with the ability to combine information from different systems.
- The candidate must be proficient in English; additional language skills are a plus.
This Regulatory Affairs Job at Astellas Careers in Bengaluru offers an exceptional opportunity to advance your Biology Career while gaining hands-on experience in global CMC submissions and regulatory compliance. Ideal for candidates from Microbiology Jobs or other life science backgrounds, this role empowers you to work on cutting-edge pharma projects, collaborate with international teams, and contribute to the delivery of safe and effective medicines worldwide. Take this chance to grow professionally, enhance your regulatory expertise, and secure a meaningful career in the life sciences sector.

































