QA/QC Career Kickstarter Webinar – Master the Skills Employers Demand in Biopharma
An exclusive webinar designed to equip aspiring biopharma professionals with in-demand QA/QC skills, industry insights, and career growth strategies.
- Date: 4th September 2025
- Time: 7:00 PM – 8:00 PM IST
- Location: Bengaluru, Karnataka (Online)
BioTecNika Hosts QA/QC Career Kickstarter Webinar – Master the Skills Employers Demand in Biopharma.
Bengaluru, 4th September 2025 – BioTecNika, India’s leading Life Sciences education and training platform, successfully organized its flagship webinar “QA/QC Career Kickstarter – Master the Skills Employers Demand in Biopharma.”
The one-hour live event attracted students, graduates, and professionals eager to explore careers in Quality Assurance (QA) and Quality Control (QC) — two of the most critical pillars of the Biopharma industry. With global demand for safe, effective biologics, biosimilars, and vaccines rising, the session provided attendees with a comprehensive roadmap to acquire the skills, tools, and insights needed to thrive in this high-growth field.
Setting the Stage – Welcome & Introduction
The webinar opened with Ms. Tithi Saha, who warmly welcomed participants and set the tone for the evening. She highlighted how QA/QC ensures patient safety and product consistency, making it the backbone of biopharmaceutical innovation.
Ms. Saha explained that careers in QA/QC are not only recession-proof but also globally competitive, as industries increasingly prioritize compliance, regulatory adherence, and quality excellence.
She then introduced the panel of expert speakers:
- Dr. Ganeshan, Scientific Expert, Regulatory Affairs & QA/QC, BioTecNika.
- Mr. Kundan Baranwal, QC Manager, Anthem Biosciences.
- Mr. Awadhesh Singh, Senior Manager, QA, Aurobindo Pharma.
Session 1: Introduction to QA/QC in Biopharma
Speaker: Dr. Ganeshan (Internal Expert)
Dr. Ganeshan began by clearly distinguishing QA vs. QC roles, noting that while QC focuses on laboratory testing and analysis, QA ensures processes and systems remain compliant and efficient.
She emphasized that together, QA and QC safeguard the entire drug development and manufacturing process, ensuring every batch of medicine reaching patients is safe and effective. Drawing from her experience in biosimilars, medical devices, and diagnostic kit development, she shared practical workflows and real-world case studies.
Key highlights:
- QA/QC as the foundation of Biopharma.
- Core responsibilities across manufacturing pipelines.
- Real-world applications in medical diagnostics and biosimilars.
Session 2: Skills & Tools Employers Demand
Speaker: Mr. Kundan Baranwal, QC Manager, Anthem Biosciences
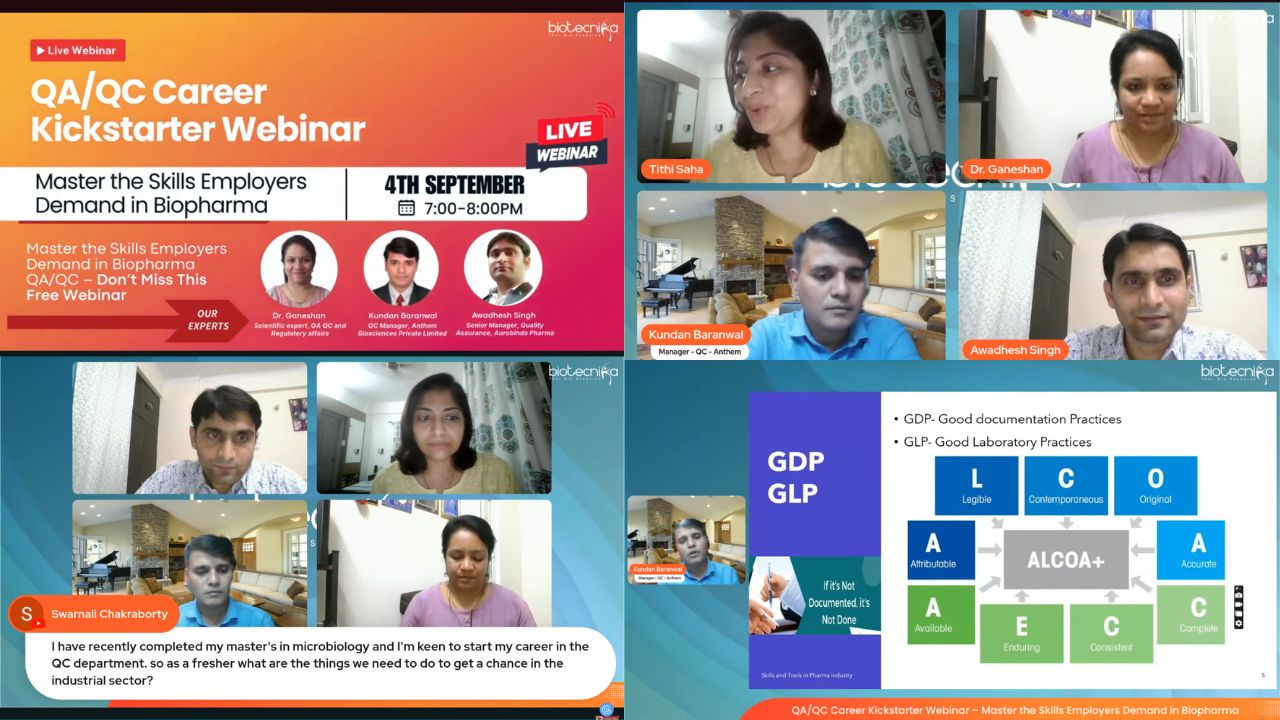
Mr. Kundan Baranwal delivered a powerful, career-focused session detailing the skills, tools, and certifications employers value most in QA/QC candidates.
He stressed that professionals must master both technical competencies and soft skills to stand out in today’s competitive market.
Highlights from his talk:
- Technical skills: Deep knowledge of GMP, GLP, SOPs, audits, compliance, documentation, and analytical techniques.
- Instrumentation expertise: Hands-on proficiency in HPLC, GC, GC-MS, FTIR, UV spectrophotometry, and Particle Size Analysis.
- Soft skills: Attention to detail, regulatory mindset, collaboration, and effective communication.
- Certifications: ISO standards, internal auditing, and advanced analytical training to boost employability.
His session was praised by participants for its practicality and clarity, offering a clear checklist of skills required to break into — and excel in — the field.
Session 3: Career Pathways & Growth in QA/QC
Speaker: Mr. Awadhesh Singh, Senior Manager, QA, Aurobindo Pharma
Mr. Awadhesh Singh concluded the expert talks with a forward-looking session on career progression in QA/QC.
He mapped out the typical career ladder — from entry-level roles like Quality Control Analyst or QA Associate to leadership positions such as Manager, Senior Manager, and Head of Quality Operations.
Drawing from his rich industry experience, he shed light on:
- Salary trends in India and abroad.
- Expanding opportunities in biosimilars, aseptic formulations, and sterile/non-sterile facilities.
- The critical role of ISO certifications in career advancement.
He shared insights from leading greenfield projects at Aurobindo Pharma, where he successfully set up sterile facilities, dry powder injection plants, and QC labs from design to commercialization.
His message resonated strongly: a QA/QC career is not just a profession, but a commitment to ensuring quality excellence and safeguarding human lives.
About the Speaker

Mr. Kundan Baranwal is an accomplished Quality Control Manager at Anthem Biosciences, bringing over 18 years of experience in pharmaceutical quality management. His career spans leadership roles at industry giants such as Biocon and Cipla, where he contributed extensively to regulatory affairs, customer technical support, and QC operations.
At Anthem, he leads QC teams handling Active Pharmaceutical Ingredients (APIs), ensuring compliance with stringent regulatory standards. His expertise covers a broad spectrum of analytical instruments including HPLC, GC, GCMS, FTIR, UV, and SOR, reflecting his technical depth and precision-driven approach.
Beyond technical mastery, Mr. Barnwal is widely respected for his team leadership, communication skills, and regulatory acumen. His career reflects a consistent ability to uphold quality systems and operational excellence in highly regulated environments. For young professionals, his journey stands as a powerful example of how deep technical skills, combined with a compliance-first mindset, can open doors to senior leadership roles in QA/QC

Mr. Awadhesh Singh is a Senior Manager of Quality Assurance at Aurobindo Pharma, with more than 17 years of experience in pharmaceutical QA leadership. He has successfully spearheaded multiple greenfield projects, including the establishment of sterile and non-sterile facilities, dry powder injection plants, and QC laboratories.
Currently, he leads QA operations for Avibactam & Aztreonam lyophilized facilities, managing a large team of 54 professionals. His hands-on leadership ensures both operational excellence and strict compliance with international regulatory standards.
Mr. Singh is also a certified internal auditor in ISO 9001, ISO 14001, and ISO 45001, which underlines his commitment to quality, environmental sustainability, and occupational safety.
He is widely recognized for building high-performing teams, driving compliance culture, and delivering results under regulatory scrutiny. For professionals aspiring to leadership roles, his career exemplifies how technical expertise, regulatory knowledge, and people management converge to create impactful QA/QC leadership.
Interactive Q&A Session
The live Q&A allowed participants to seek personalized advice on certifications, transitioning from academia, and breaking into QA/QC roles. The speakers provided actionable, experience-backed responses, leaving attendees with clear next steps for career advancement.
Course Launch – BioTecNika’s QA/QC Career Program
The event also marked the launch of BioTecNika’s QA/QC Career Program for Biopharma & Medical Devices, designed to make aspirants industry-ready.
Program features include:
- Hands-on QA/QC modules.
- Mentorship by industry leaders.
- Resume and interview workshops.
- Certifications and project-based learning.
To add further value, all attendees received a Freebie: “Biopharma & Medical Device QA/QC Career Path: From Compliance to Quality Excellence (ebook)“
Key Insights from the Webinar
- QA/QC is the foundation of Biopharma success.
- Technical skills (GMP, SOPs, instrumentation) and soft skills (regulatory mindset, communication) are equally essential.
- Certifications and hands-on training significantly enhance employability.
- Clear career pathways exist from entry-level roles to senior leadership.
- Global demand for QA/QC professionals continues to rise, with competitive salaries.
About BioTecNika
BioTecNika is a leading Life Sciences education and training platform dedicated to empowering students, researchers, and professionals with industry-relevant skills and mentorship. Through expert-led courses, workshops, and certification programs, BioTecNika bridges the gap between academic learning and global industry demands.
With a mission to make world-class training accessible to all, BioTecNika has helped thousands of learners secure rewarding careers in Biotechnology, Pharmaceuticals, Clinical Research, and Regulatory Affairs.
Call to Action
BioTecNika invites all Life Sciences aspirants to join its QA/QC Career Program and accelerate their journey toward impactful careers in Biopharma and Medical Devices.
For details on this program and upcoming training opportunities, visit: www.biotecnika.org



























Hi my name is deepak Sonti education in graduation complete bsc biotechnology from Vikram University Ujjain