Clinical Data Management 360°: Skills & Techniques to Get into Leading Job Roles
- Date: 20th August 2025
- Time: 7:00 PM – 8:00 PM IST
- Location: Bengaluru, Karnataka (Online)
BioTecNika successfully organized its flagship webinar “Clinical Data Management 360°: Skills & Techniques to Get into Leading Job Roles” on August 20, 2024, a highly anticipated knowledge-sharing event that attracted students, fresh graduates, and professionals eager to explore career opportunities in the expanding field of Clinical Data Management (CDM).
The one-hour live session brought together leading experts who highlighted the scope, skills, and global opportunities available in CDM. Participants gained a comprehensive 360° view of the field, from the fundamentals of CDM to advanced skills, tools, and career strategies required to secure leading job roles in Pharmaceuticals, Biotechnology, Contract Research Organizations (CROs), and Healthcare IT.
Setting the Stage – Welcome & Introduction
The Clinical Data Management 360° webinar commenced with a warm introduction by Dr. Tanushree, who welcomed attendees and set the context for the evening. She emphasized the importance of Clinical Data Management as a rapidly growing discipline within Clinical Research and Drug Development. In her opening remarks, she noted how CDM professionals serve as the backbone of Clinical Trials, ensuring that high-quality, accurate, and reliable data support life-saving drug discoveries.
Dr. Tanushree highlighted the rising global demand for skilled CDM professionals and introduced the session as a unique opportunity to gain expert insights into building a successful career in this field.
- CDM Uncovered – What, Why & Where?
The first session was led by Mrs. Bhanu Melvin, Academic & Research Support Specialist in CDM, Pharmacovigilance, and Regulatory Affairs, who brought a wealth of Academic, Healthcare, Regulatory, and training experience to the session.

Mrs. Melvin is an accomplished professional with a strong academic foundation in Pharmacy and over a decade of dynamic experience across Academia, Healthcare, Regulatory Affairs, and corporate training. She has successfully trained more than 1,500 individuals, ranging from academic aspirants to working professionals, in areas including Clinical Research, Pharmacovigilance, Clinical Data Management, Regulatory Affairs, and Clinical SAS Programming.
She also holds globally recognized certifications in Pharmacovigilance, Lean Six Sigma Black Belt, Clinical Research, Clinical SAS Programming, and ISO 9001:2015 Lead Auditing. Her multidisciplinary exposure has allowed her to bridge the gap between Scientific knowledge, Regulatory frameworks, and operational excellence. Passionate about delivering transformative training, she has consistently designed curricula and solutions that respond to the evolving demands of the Life Sciences industry.
Session Highlights
Mrs. Melvin’s session introduced attendees to the foundations of Clinical Data Management, its role in Clinical Research, and its vast career potential. Key topics included:
- What is Clinical Data Management?
A clear explanation of CDM and its importance in ensuring data accuracy, consistency, and reliability in Clinical Trials.
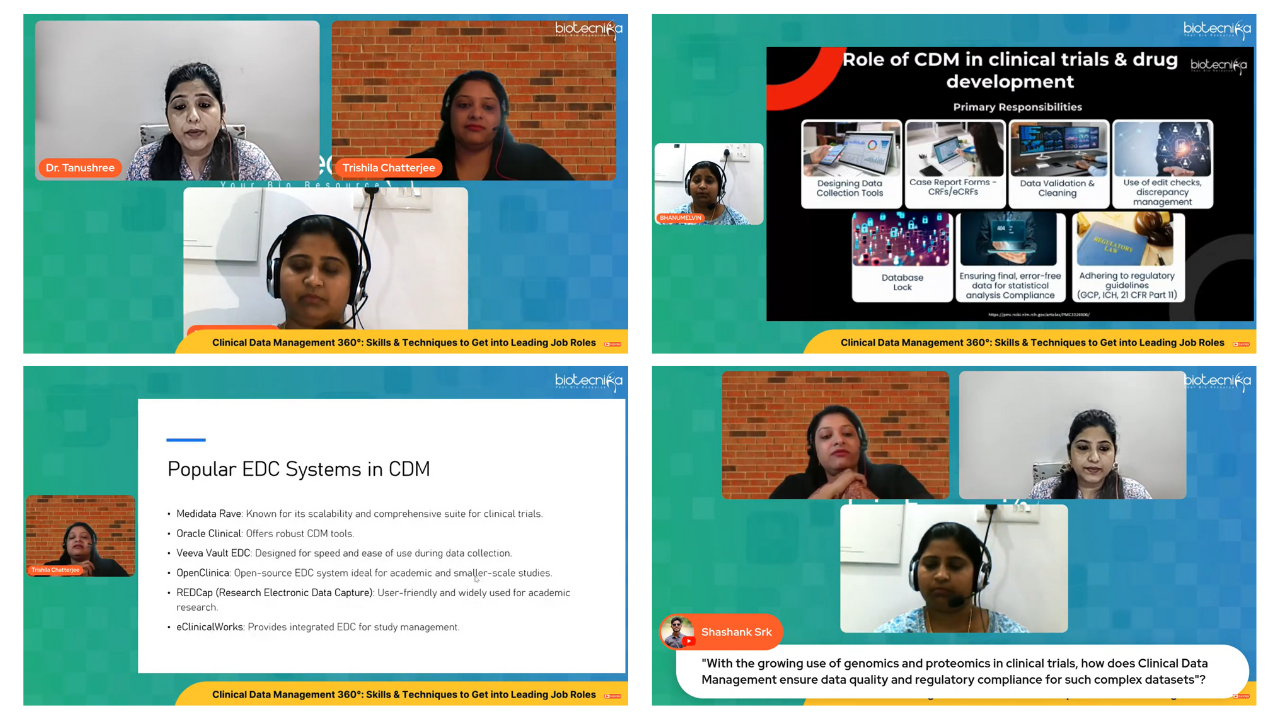
- The Role of CDM in Clinical Trials & Drug Development
How CDM professionals play a vital role in ensuring that new drugs meet regulatory standards, contributing to safer and more effective treatments.
- Industry Relevance & Stakeholders
The involvement of pharmaceutical companies, CROs, Biotech firms, and Healthcare IT organizations in CDM operations.
- Career Opportunities in India & Abroad
An overview of career paths ranging from entry-level positions such as Data Coordinator to senior roles like Data Manager and Medical Coder. Salary trends in India and international markets were also discussed.
- How BioTecNika Supports Career Growth
Attendees learned about BioTecNika’s specialized training programs, mentorship, and placement support designed to accelerate career journeys in CDM.
Her session laid a strong foundation for participants, equipping them with a clear understanding of the significance of CDM in today’s Clinical Research ecosystem.
- Core Skills & Must-Know Tools for CDM Careers
The second session was delivered by Ms. Trishila Chatterjee, Lead Data Manager at Novartis India, who brought over 12 years of extensive experience in Clinical Data Management.

Ms. Chatterjee is a Data Management professional currently serving as Lead Data Manager at Novartis India. With more than a decade of experience across Phase I–IV clinical trials, she has mastered all stages of CDM, including study setup, conduct, and closeout.
Her expertise lies in project management, oversight of multiple Clinical Trials, and ensuring data quality through collaboration with cross-functional teams and vendors. She has led mega trials, managed large teams, and excelled in risk-based monitoring models.
With over seven years of specialization in Oncology, her therapeutic expertise also spans Cardio-renal, Immunology, Metabolic disorders, Women’s Health Sciences, and Endocrinology. Recognized as a key team player, she is widely respected as a subject matter expert and mentor within the CDM field.
Session Highlights
Ms. Chatterjee’s presentation provided participants with practical strategies to develop the skills and expertise necessary to thrive in CDM roles. Key areas included:
- Core Skills Knowledge
Emphasis on essential CDM skills such as Good Clinical Practice (GCP), data entry, query management, and adherence to Standard Operating Procedures (SOPs).
- Technical Tools Mastery
Insights into widely used Electronic Data Capture (EDC) systems such as Oracle Clinical, Medidata Rave, and OpenClinica.
- Databases & Regulatory Knowledge
A deep dive into CDISC, MedDRA, WHO-DD databases, and global Regulatory standards, including FDA and ICH-GCP guidelines.
- Career Readiness
Practical tips for crafting a CDM-focused resume, leveraging professional networking, and excelling in job interviews.
- Upskilling Strategies
Guidance on certifications, workshops, live projects, and hands-on training opportunities that enhance employability.
Her session resonated with participants, particularly those eager to understand the technical and Regulatory landscape of CDM, while also providing a clear roadmap for career success.
Interactive Q&A Session
The webinar concluded with an engaging Q&A session, during which attendees had the opportunity to interact directly with the speakers. Questions ranged from how to choose the right CDM certification to practical advice on transitioning from academia to industry. Both speakers provided valuable insights, leaving participants with actionable steps to take forward.
Key Insights from the Clinical Data Management 360° Webinar
The event offered numerous takeaways that highlighted the importance and career potential of Clinical Data Management.
- Clinical Data Management is crucial for the success of Clinical Trials and Drug Development.
- The demand for skilled CDM professionals is growing globally, creating significant career opportunities.
- Core skills in GCP, SOPs, and data handling are non-negotiable for success in CDM.
- Technical proficiency in tools such as Oracle Clinical, Medidata Rave, and OpenClinica distinguishes job-ready professionals.
- Familiarity with Regulatory frameworks and global standards ensures compliance and career competitiveness.
- Certifications, live projects, and workshops dramatically increase employability.
- Building a CDM-focused resume and networking strategically are vital for job seekers.
About BioTecNika
BioTecNika is a pioneering Life Sciences education and training platform dedicated to empowering students, researchers, and professionals with the knowledge and skills required to excel in Biotechnology, Clinical Research, Bioinformatics, and allied domains. Through its expert-led courses, certification programs, and industry-focused workshops, BioTecNika has helped thousands of learners bridge the gap between academic knowledge and industry readiness.
The organization is dedicated to cultivating the next generation of Scientific professionals by offering cutting-edge resources, mentorship, and placement support. Its mission is to make world-class training accessible to all, thereby shaping the future of Healthcare and Life Sciences.
Call to Action
The webinar also announced the launch of BioTecNika’s Clinical Data Management Training Program, scheduled to begin on August 25, 2024. This comprehensive course is designed to equip participants with hands-on experience, certifications, and project-based learning opportunities, preparing them for top-tier roles in CDM.
For more details on the program and upcoming events, visit: www.biotecnika.org























This CDM webinar by BioTecNika is extremely practical! From basic concepts to advanced tools, to salary trends and resume tips, even experts from Novartis shared practical experience. Whether you are a student or someone looking to switch careers, you can find a clear career direction and gain a lot.