Clinical Data Management Webinar: A Fast-Growing Career in Life Sciences
Date: 30/05/2025
Location: Bengaluru, Karnataka, India
BioTecNika recently conducted an engaging live webinar titled “Clinical Data Management as a Career – Roles, Skills & Industry Insights.” Hosted by Dr. Tanushree Saxena, the session featured seasoned experts from the Clinical Research industry. It focused on the growing importance of Clinical Data Management (CDM) in Drug Development and career opportunities in this high-demand sector. The session is now available for replay on YouTube and continues to inspire budding Life Science professionals across India.
Understanding the Role of CDM in Clinical Trials and Drug Development
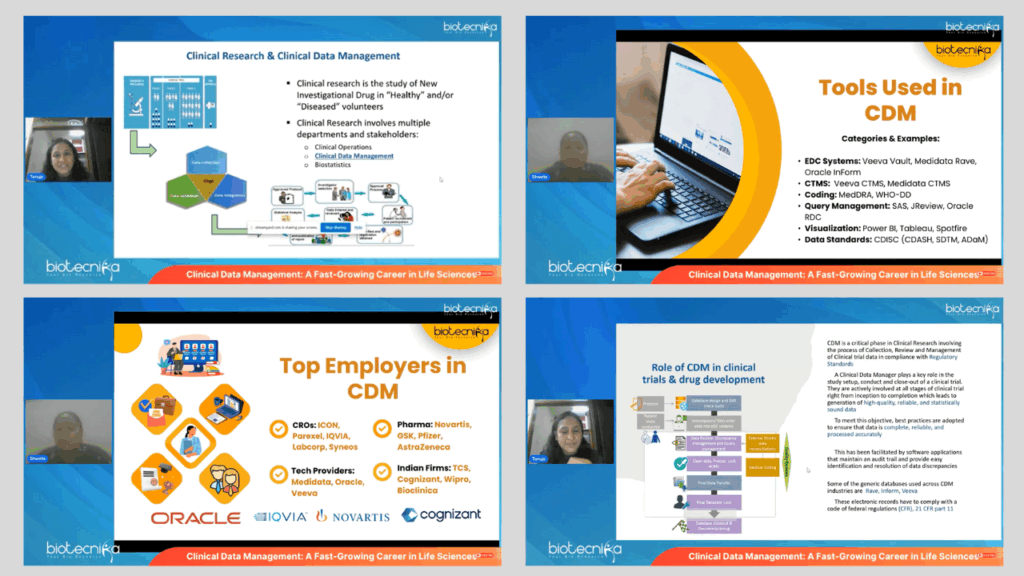
The webinar offered attendees a thorough and insightful overview of Clinical Data Management and its crucial contribution to the success of Clinical Trials. The sessions detailed how CDM ensures that all trial data is accurately collected, validated, and maintained in strict compliance with Regulatory Standards. Emphasis was placed on the pivotal role CDM professionals play in safeguarding the accuracy and reliability of data, which directly impacts patient safety and the scientific integrity of Clinical Research. The speakers also underscored how effective Data Management supports evidence-based Drug Development, enabling timely and well-informed decisions throughout the drug approval process. Overall, the webinar highlighted CDM as an essential backbone of Clinical Trials that bridges operational excellence with Regulatory Compliance and Scientific rigor.
Key Highlights from the Webinar
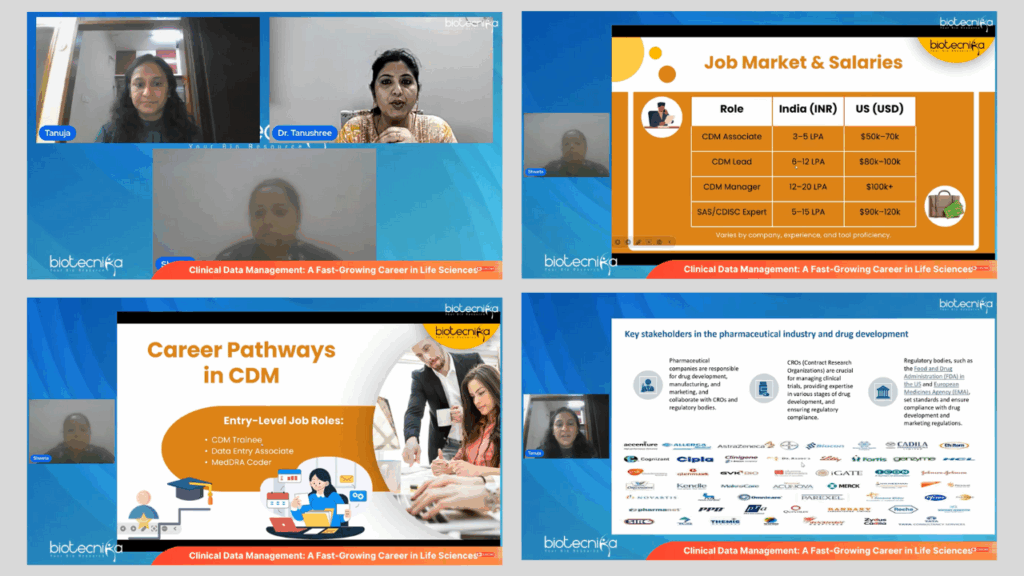
- Insightful introduction to the CDM Lifecycle – From study setup and data collection to data cleaning and trial close-out.
- Demonstration of CDM tools – Including Electronic Data Capture (EDC) systems like Medidata Rave and Oracle Inform, as well as Clinical Trial Management Systems (CTMS).
- Industry shift towards automation and AI (Artificial Intelligence) – Showcasing how advanced technologies are streamlining Clinical Data handling, improving data quality, and reducing time to market for therapies.
- India’s growing CDM market – Especially noteworthy in light of global outsourcing trends and the rise in clinical trial activity across Asia-Pacific.
- Career mapping and skill-building strategies – With actionable guidance for students and professionals seeking to enter or advance in the field.
Webinar Insights from Industry Leaders

Ms. Fernandes delivered an in-depth session on the relevance of CDM in the global clinical research landscape. She covered the end-to-end responsibilities of CDM professionals, from designing case report forms to resolving data discrepancies. Her talk emphasized regulatory compliance, data integrity, and the interdisciplinary nature of CDM. She also addressed common misconceptions and presented the career potential for life science graduates in India and abroad.

Ms. Shweta offered a practical perspective on the tools, qualifications, and certifications necessary to build a rewarding career in CDM. Drawing from her experience, she shared strategies on how to get started in the industry, the importance of internships, GCP training, and how to grow from an entry-level associate to a CDM lead. Her session concluded with tips on resume building, interview preparation, and Biotecnika’s training support.
Expert Speakers
Ms. Tanuja Fernandes – Director, Fortrea
With over 20 years in clinical research, Ms. Tanuja Fernandes is a seasoned expert in clinical data management, trial operations, and regulatory compliance. Currently serving as Director at Fortrea, she has previously held leadership roles at Labcorp, Covance, Parexel, Cognizant, and Accenture. Her extensive experience spans managing global teams, overseeing multi-phase clinical trials, and ensuring data quality and regulatory alignment. Ms. Fernandes is known for her strategic insights and commitment to advancing CDM practices in India.
Ms. Shweta Birajdar– Ms. Shweta is a skilled CDM professional and trainer at BioTecNika, known for her practical, learner-focused teaching. She has trained hundreds in GCP, Data Validation, Medical Coding, and EDC tools. Her session covered essential skills for CDM careers, growth pathways, certification benefits, and how BioTecNika supports students with resume building, mock interviews, and placement assistance.
Catch up on the webinar here:
About BioTecNika
BioTecNika is a premier Biosciences education platform that provides cutting-edge training and career development in Life Sciences, Biotechnology, and Clinical Research. Through expert-led courses, webinars, and mentoring, BioTecNika empowers students and professionals to stay ahead in competitive scientific industries such as Clinical Data Management, Pharmacovigilance, Bioinformatics, and more.
For those interested in pursuing a career in Clinical Data Management, BioTecNika’s next CDM Training Program begins on 10th June 2025. Early registration is recommended due to limited seats and high demand!
Basics to Advanced – With Live Projects & Placement Assistance!
Next Batch Starts: June 10, 2025