Mind-Controlled Prosthetic Robot Arm to Help Amputees Developed
A new study by neuroscientists at the University of Chicago shows how amputees can learn to control a robotic arm through electrodes implanted in the brain.
The study details changes that take place in both sides of the brain — important because the side of the brain opposite to a limb is the one that controls it. The research shows how both areas can create new connections to learn how to control the device, even several years after an amputation.
“That’s the novel aspect to this study, seeing that chronic, long-term amputees can learn to control a robotic limb,” said Prof. Nicho Hatsopoulos, PhD, who served as senior author of the study.
Funded by the Defense Advanced Research Projects Agency and spurred by the return of injured Iraq and Afghanistan war veterans, the research aims to design prostheses that will not only be able to move, but will also provide amputees and quadriplegics with a sense of touch.
Scientists have known for more than a century that applying electricity to neurons can elicit certain reactions — a muscle twitch, a sudden feeling of euphoria, a long-forgotten memory recalled. But stimulating those cells to help people overcome certain disabilities has been done only more recently, spearheaded in the 1960s by the development of the cochlear implant for hearing.
The scientists set out to identify and replicate the qualities of touch, including texture, shape and force, through complex mathematical equations known as algorithms.
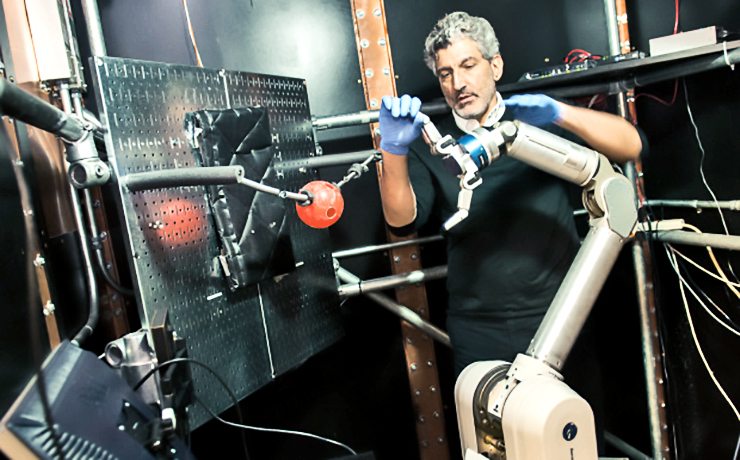
The study involved three rhesus monkeys who suffered injuries at a young age and had to have an arm amputated to rescue them four, nine and 10 years ago, respectively. Their limbs were not amputated for the purposes of the study. In two of the animals, the researchers implanted electrode arrays in the side of the brain opposite, or contralateral, to the amputated limb. This is the side that used to control the amputated limb. In the third animal, the electrodes were implanted on the same side, or ipsilateral, to the amputated limb. This is the side that still controlled the intact limb.
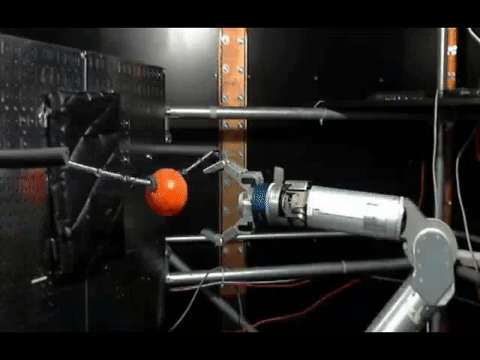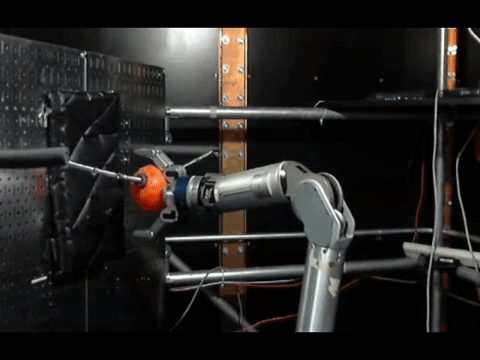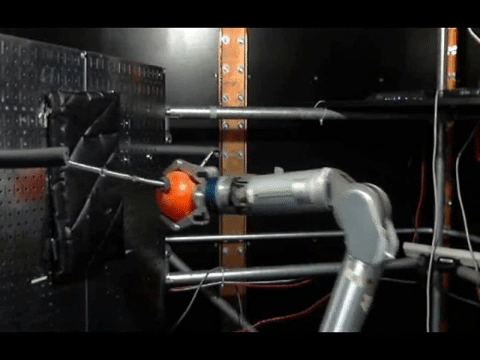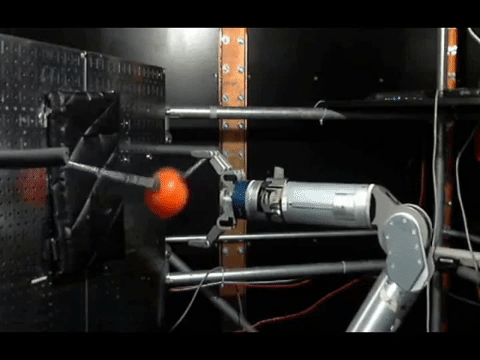
The animals were then trained to move a robotic arm and grasp a ball using only their thoughts. The neurons activity was controlled by the scientists where the electrodes were placed, and used a statistical model to calculate how the neurons were connected to each other before the experiments, during training and once the monkeys mastered the activity.
The connections between neurons on the contralateral side—the side that had been controlling the amputated arm—were sparse before the training, most likely because they had not been used for that function in a long time. But as training progressed, these connections became more robust and dense in areas used for both reaching and grasping.
On the ipsilateral side—the side that had been controlling the monkey’s intact arm—the connections were dense at the beginning of the experiments. But the researchers saw something interesting as training progressed: first the connections were pruned and the networks thinned, before rebuilding into a new, dense network.
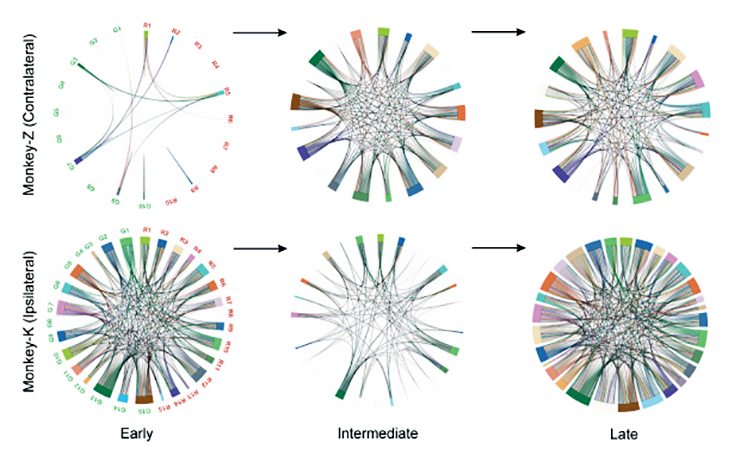
“That means connections were shedding off as the animal was trying to learn a new task, because there is already a network controlling some other behavior,” said Karthikeyan Balasubramanian, PhD, a postdoctoral researcher who led the study. “But after a few days it started rebuilding into a new network that can control both the intact limb and the neuroprosthetic.”
The team plans to continue their work by combining it with research by other groups, in order to equip neuroprosthetic limbs with sensory feedback about the sense of touch and the sense of proprioception, or where the limb is located in space.