Dissociating Side Effects of Combat Opined Addiction
The “wonder drug” pain medications of the mid-1990s have turned out to be a major problem – and a big disappointment.
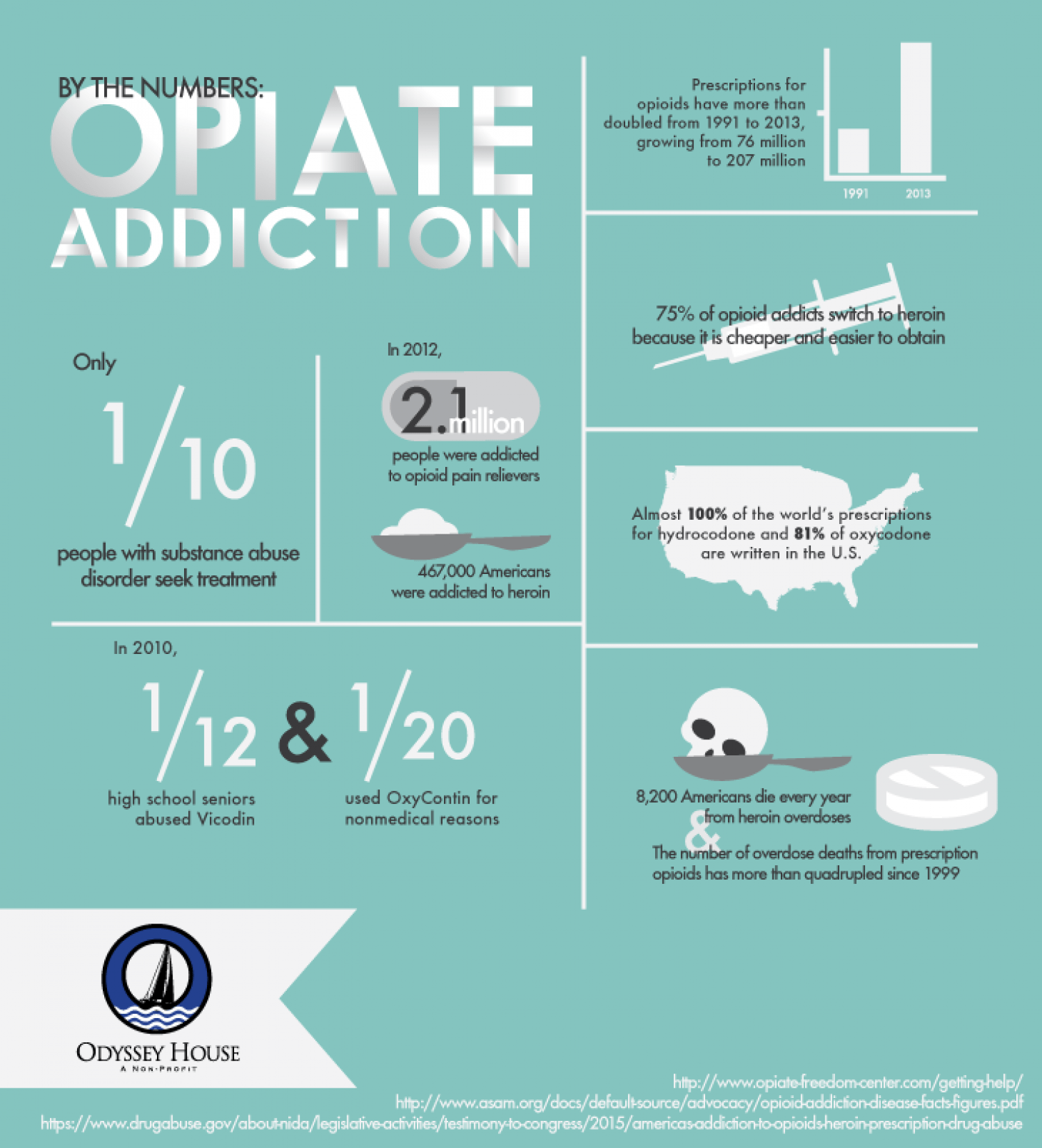
More than 40 Americans die each day from prescription opioid overdoses according to a U.S. Centers for Disease Control and Prevention report.
Opioid medications, sometimes known as pain relievers, are the most widely prescribed class of drugs worldwide. Opioid pain relievers are generally safe when taken for a short time and as prescribed by a doctor, but because they produce euphoria in addition to pain relief, they can be misused (taken in a different way or in a larger quantity than prescribed, or taken without a doctor’s prescription). Regular use—even as prescribed by a doctor—can lead to dependence and, when misused, opioid pain relievers can lead to overdose incidents and deaths.
In the face of increasing rates of addiction and related deaths, scientists have now discovered a way to separate two effects of opioids- pain relief and breathing — opening a window of opportunity to make effective pain medications without the risk of respiratory failure.
Opioid medications suppress pain by binding to specific receptors (proteins) in the brain; these same receptors also produce respiratory suppression. However, the way these receptors act to regulate pain and breathing may be fundamentally different.
Previous studies have demonstrated that avoiding one particular signaling pathway in animal models led to more favorable responses to morphine (pain relief without respiration effects).
Therefore, in this National Institute on Drug Abuse (NIDA) study, investigators explored if they could make drugs that would turn on the pathways associated with pain relief and avoid the pathways associated with respiratory suppression.
“We are pleased to have uncovered a potential new mechanism to create safer alternatives to opioid medications, ones that would be far less likely to cause the side effects that lead to overdose deaths associated with the misuse of opioids,” said NIDA Director Nora D. Volkow, M.D. “We are excited that basic research on how opioid drugs work in the brain has led to this novel approach and that we continue to make critical progress in this area.”
The study showed that as the degree of bias (divergence) increased, so too did the ability of an opioid to reduce pain in mice without affecting breathing. Similarly, compounds that favor the breathing pathway produced more respiratory side effects at lower doses. Ultimately, opioids with a larger divergence (bias factor) had a larger margin of safety, or therapeutic window, opening up an opportunity for medical intervention.
“In this study, we demonstrate that this divergence, which we call biased agonism, is not an ‘all or none’ phenomenon, but rather exists as a spectrum. As such, if a small degree of divergence between pathways in cell culture produces a minimal benefit in living organisms, there is potential to chemically improve the signaling bias and subsequently improve the therapeutic safety window,” said Laura M. Bohn, Ph.D., principal investigator on the study from The Scripps Research Institute.