Cianna Medical’s Savi Scout Tumor Locator Approved by the FDA
The FDA has now cleared the world’s first non-radioactive breast tumor localization system for the long-term implant, the California-based manufacturer, Cianna Medical announced.
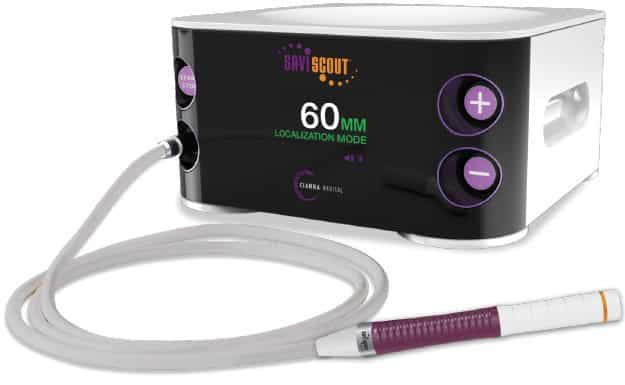
This device, the SAVI Scout reflector, is part of the SAVI Scout surgical guidance system (Cianna Medical Inc).
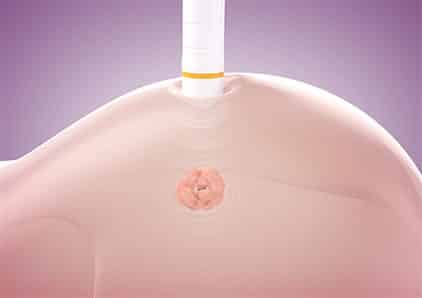
The system uses an intratumoral electromagnetic wave device, implanted under ultrasound or mammographic guidance, to localize occult breast lesions with a probe during surgery.
Because the implant doesn’t emit any harmful radiation and is a fairly simple device, the emitter can remain implanted for an indefinite period of time. This gives clinicians greater freedom of scheduling the different steps that patients have to undergo in preparation for biopsy or surgical excision.
Coupled with the rest of the SCOUT system, the 4-millimeter long implant can provide physicians its exact location down to a 1-millimeter resolution. It is detected using an electronic wand that relies on the radar as the localization modality. The wand is simply placed against the breast and the SCOUT’s controller provides audio and visual feedback pointing to the implant’s location.
Results from a pilot study evaluating successful placement, localization and retrieval of the SAVI SCOUT were presented as part of the late-breaking scientific sessions at San Antonio Breast Cancer Symposium today. The study, which included 24 patients, resulted in 100% surgical success using SAVI SCOUT. In all cases, the target tissue and reflector were successfully removed; there were no incidents of reflector migration or adverse events. Pathology reports showed clear margins in comparable numbers to radioactive seed location.
“Achieving this milestone significantly advances Cianna Medical’s mission to reduce the burden breast cancer treatment places on women and their families. I applaud the vision of all our physicians and health systems who are leading the way in offering a new standard of care in breast tumor localization,” in a press statement said Jill Anderson, President and CEO of Cianna Medical. “SCOUT has become the most precise and broadly applicable non-wire localizing system in the industry and more than 170 leading medical centers across the United States have adopted SCOUT Radar Breast Localization and Surgical Guidance System as an alternative to placing wires on the day of surgery.”
“We believe that SAVI SCOUT provides two major advantages: First, the reflector can be comfortably placed several days prior to surgery, on the day of surgery by a radiologist or in the operating room by a surgeon. Second, the SAVI SCOUT handpiece can be used with retractors so, as the dissection proceeds, we receive immediate, real-time guidance for the lumpectomy,” said Pat Whitworth, M.D., Director, Nashville Breast Center, Nashville, Tenn. “We are finding SAVI SCOUT eliminates the need to stop for intraoperative ultrasound; we can now use imaging only before and after the resection. I expect it’s going to be a welcome advance for surgeons and patients.“