Bausch + Lomb and Nicox Announce FDA Approval of Vyzulta
Vizsla, the first prostaglandin analog with one of its metabolites being nitric oxide (NO), has now been approved by the FDA for the reduction of intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
Valeant Pharmaceutical’s wholly owned subsidiary, Bausch + Lomb, a leading global eye health company, and Nicox, an international ophthalmic company, has announced the approval.
“Vyzulta represents the first FDA-approved therapy developed through our proprietary NO-donating research platform,” Michele Garufi, chairman, and CEO of Nicox said in a company news release. “We look forward to continuing to leverage our platform in the development of additional innovative ophthalmic compounds.”
Following topical administration, VYZULTA, a once-daily monotherapy with a dual mechanism of action, works by metabolizing into two moieties, latanoprost acid, which primarily works within the uveoscleral pathway to increase aqueous humor outflow, and butanediol mononitrate, which releases NO to increase outflow through the trabecular meshwork and Schlemm’s canal.
The most common ocular adverse events include conjunctival hyperemia, eye irritation, eye pain and installation site pain. Increased pigmentation of the iris and periorbital tissue and growth of eyelashes can occur.
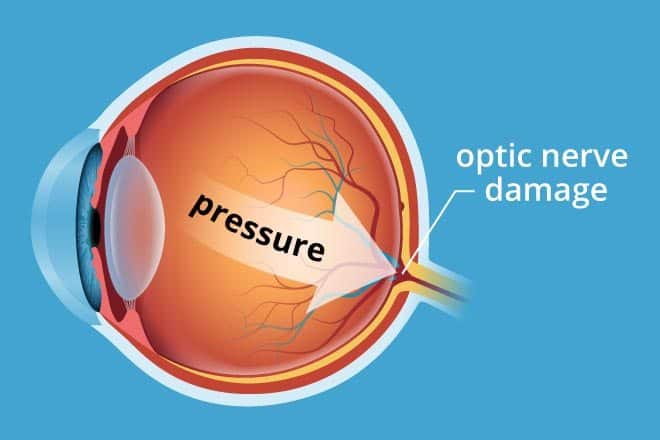
In glaucoma patients, damage to the trabecular meshwork, through
which the majority of the aqueous humor passes, can lead to reduced drainage and as a result, elevated IOP. Lowering IOP, even in patients with normal baseline levels, can delay, or even prevent damage to optic nerves, helping to reduce the risk of glaucomatous visual field loss.“Vyzulta, like other prostaglandin analogs, is a once-daily monotherapy. However, unlike other prostaglandin analogs, it has a dual mechanism of action,” Robert N. Weinreb, MD, chairman, and distinguished professor of ophthalmology and director, Hamilton Glaucoma Center at the University of California San Diego, said. “It works by metabolizing two moieties – latanoprost acid and butanediol mononitrate – which each target a specific pathway.”
“Latanoprost-acid is well known to ophthalmologists. It’s an F2-alpha prostaglandin analog, and primarily works within the uveoscleral pathway to increase aqueous humor outflow, so it’s increasing outflow through the nonconventional pathway. The second, butanediol mononitrate, releases nitric oxide, to increase outflow through the trabecular meshwork and Schlemm’s canal, and this clearly differentiates it from other prostaglandin analogs that are being used to treat glaucoma,” Dr. Weinreb added.
Preclinical studies have shown that NO plays a role in controlling IOP in normal eyes by increasing aqueous humor outflow through the trabecular meshwork and Schlemm’s canal. Studies have also demonstrated that patients with glaucoma have reduced levels of NO signaling in their eyes, providing a rationale for the therapeutic value of NO-releasing molecules for patients with open-angle glaucoma or ocular hypertension.
As a result of this approval, NicOx will receive a $17.5 million from Bausch + Lomb and will make a $15 million payment to US pharma giant Pfizer under a previous license agreement.
Joseph Papa, chairman and chief executive of Valeant, said: “With today’s approval of Vyzulta, our customers and their patients with glaucoma now have a new treatment option that can help provide consistently and sustained IOP lowering, the only modifiable risk factor that can help slow down the progression of the disease.”