Polarity Announces U.S FDA Registration Of Skin-Regeneration Platform
PolarityTE, the regeneration medicine outfit, has now announced the Federal registration of lead candidate SkinTE, an autologous construct intended for homologous uses of skin tissues.
As an FDA-registered HCT/P, SkinTE may now be made available for appropriate human use in the United States. PolarityTE has initiated a controlled, limited-market release of the product to select medical institutions, and expects to accelerate commercialization in 2018 as the company scales up manufacturing efforts.
Launched earlier this year, the platform developed at PolarityTE allows the company’s products to regenerate a patient’s tissues using their own cells. The company’s first product, SkinTE, is being optimized for clinical use in skin regeneration, with all layers, hair and appendages.
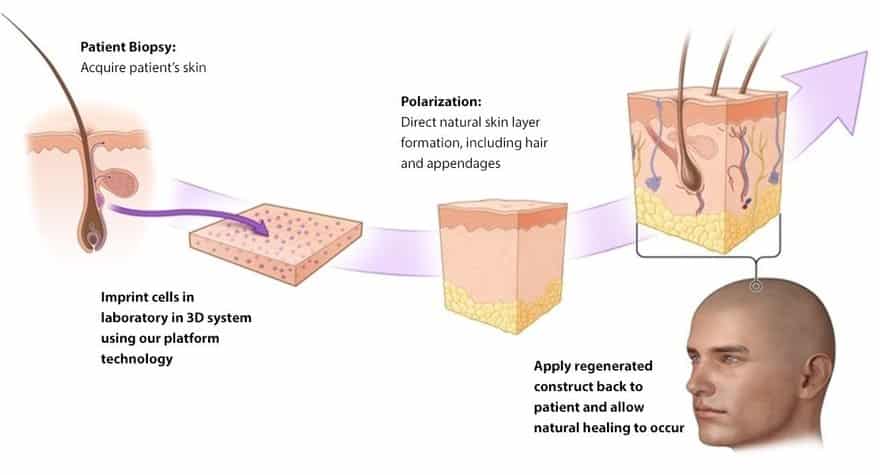
In preclinical studies, SkinTE demonstrated full-thickness regenerative healing, hair follicle formation, immediate and complete wound coverage, as well as the progressive regeneration of all skin layers, said Dr. Edward Swanson, PolarityTE’s chief operating officer.
“The FDA registration of SkinTE is an important milestone that enables us to bring this revolutionary technology into clinical practice,” he said. “This achievement enables us to deliver our new and pragmatic solution for skin regeneration, and to change the face and practice of regenerative medicine toward patient-tailored tissue products.”
“The FDA registration of SkinTE is an important regulatory step that sets the stage for commercialization and a staged market entry of this revolutionary technology into clinical application,” said Denver M. Lough, M.D., Ph.D., Chief Executive Officer of PolarityTE. “This achievement enables us to deliver an entirely new and pragmatic solution for skin regeneration as well as the ability to change the face and practice of regenerative medicine toward patient-tailored tissue constructs.”























