Century Old Viruses May Be Our Best Bet Against Antibiotic Resistance
The discovery that antibiotics can treat bacterial infections dramatically changed human health, and many once deadly infections are now curable. Yet often we hear about bacteria that are no longer killed effectively by antibiotics. These bacteria are known as antibiotic resistant, and they’re a growing problem in medicine.
Antibiotic resistance is not new. In fact, it existed long before people discovered how to use antibiotics as medicine. Many antibiotics are made from compounds bacteria or fungi produce to help them compete in their natural environments. This means that in nature, bacteria are under selective pressure to pass on advantages, just like they are when we treat an infection with antibiotics. Sometimes in medicine, antibiotics are used too often or incorrectly, which can cause resistance to spread faster than it would naturally.
Resistance to antibiotics is becoming increasingly prevalent and threatens to undermine healthcare systems across the globe. Antibiotics including penicillins, cephalosporins, and carbapenems are known as β-lactams and are the most commonly prescribed worldwide.
And it hasn’t been easy to develop new drugs in order to stay ahead of the problem. Many major pharmaceutical companies have stopped developing new antibiotics, and the drugs that are still in development have faced numerous stumbling blocks toward approval.
So some drugmakers are starting to turn to other solutions, including one that’s actually had a fairly long history: phage therapy.
“There’s huge potential there that regular antibiotics don’t have,” NYT columnist Carl Zimmer told Business Insider in 2015. “I think what we’d actually have to work on is how we approve medical treatments to make room for viruses that kill bacteria.”
Dr. Paul Grint, CEO of one small company, AmpliPhi Biosciences, is trying to turn phage therapy into a tool that doctors might be able to one day use alongside antibiotics to treat serious infections. The company’s working on phage-based treatments to treat Staphylococcus aureus, a bug implicated in sinus infections, and Pseudomonas aeruginosa, a bug connected to lung infections in people with cystic fibrosis.
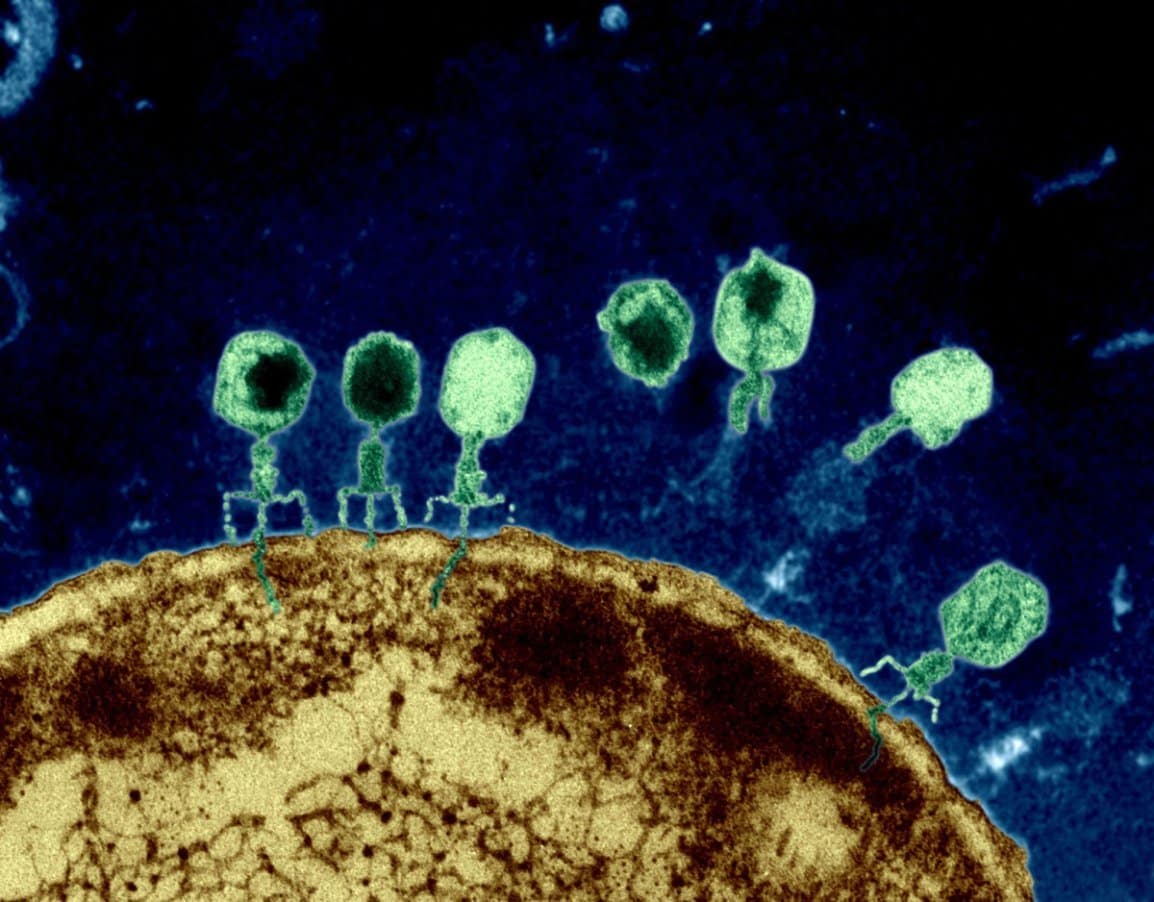
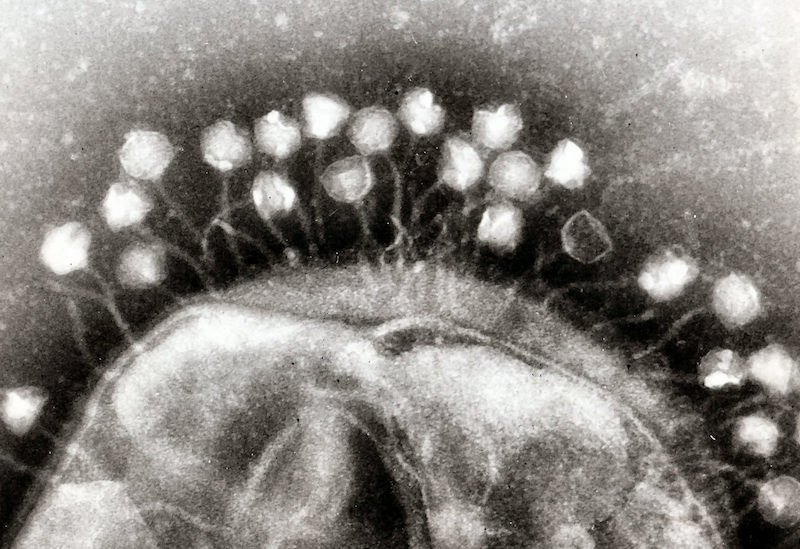
Bacteriophages (phages) are naturally occurring viruses that selectively kill bacteria, including antibiotic-resistant bacteria. Phages are the most abundant organisms on Earth and have evolved not only to kill bacteria directly, but also to penetrate and disrupt biofilms, and have been shown to restore antibiotic sensitivity to drug-resistant bacteria.
AmpliPhi Biosciences Corporation is a clinical-stage biotechnology company focused on treating antibiotic-resistant infections using its proprietary bacteriophage-based technology. AmpliPhi’s lead product candidates target multidrug-resistant S. aureus and P. aeruginosa, which are included on the WHO’s 2017 Priority Pathogens List. Phage therapeutics are uniquely positioned to address the threat of antibiotic-resistance as they can be precisely targeted to kill select bacteria, have a differentiated mechanism of action, can penetrate and disrupt biofilms (a common bacterial defense mechanism against antibiotics), are potentially synergistic with antibiotics and have been shown to restore antibiotic sensitivity to drug-resistant bacteria.
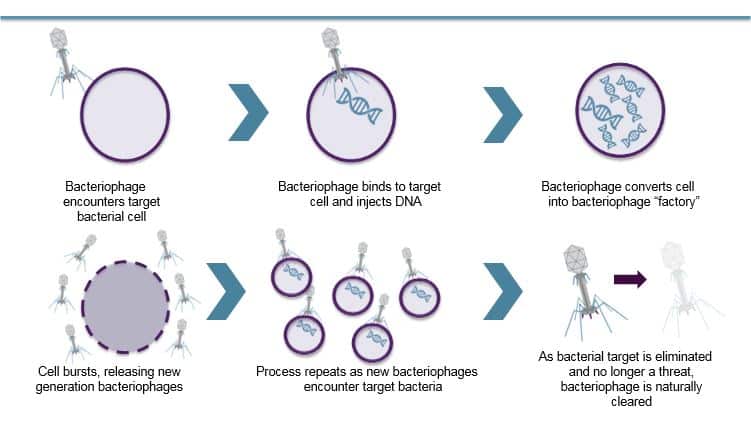
AmpliPhi’s bacteriophage-based therapeutic approach selects and optimizes bacteriophage to target and kill the most clinically-relevant bacterial strains. Due to the self-replicating nature of bacteriophages, they can rapidly increase in numbers, allowing for a relatively small input dose to generate an effective therapeutic outcome.
Once AmpliPhi’s bacteriophage mix has infected the host bacteria cell, the resulting infection reprograms the bacteria to replicate itself, producing dozens or hundreds of new bacteriophages, which causes the bacterium to burst, thereby releasing the remaining phages to similarly infect neighboring bacteria. A typical bacteriophage infection cycle takes only a few minutes or hours and only stops when the bacteriophages run out of target bacteria.
Once the bacteria have been eliminated, there is nothing left for the phage to infect, and it is removed from the body through normal clearance processes.
Currently, AmpliPhi is developing a pipeline of novel bacteriophage antibacterial therapeutics designed to be effective against antibiotic-resistant bacteria which can be delivered through multiple mechanisms for targeted therapy, including intra-nasal, topical and inhaled.
There are also some researchers like a group at the University of California at San Diego that is researching phage therapy. In 2016, for example, researchers at UCSD used AmpliPhi’s therapy to treat a professor at the university who had a drug-resistant infection.
Bacteriophages as antibacterial therapies are an exciting new development in antibiotics, but their increasing presence in the field is another nail in the coffin for organic chemistry in pharmacy. Antibiotics, once exclusively the purview of synthetic organic chemists, could soon go the way of the rest of the pharmaceutical industry and become the domain of biologists.